 |
| 120 L fermentor: Cell disruption
and purification |
| Cell
disruption |
|
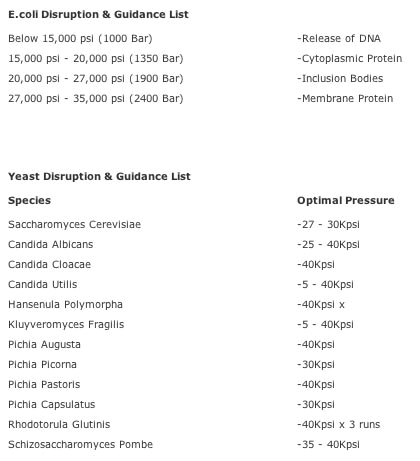
- Switch on chiller (4°C)
- switch on disruptor
- run 1-2 fillings of water through at 10-20 KPSI (don't go
beyond 40KPSI with the pressure adjustment, please)
- run your sample (foaming)
- run water through until machine is clean
- run 20% ethanol through to prevent growth
- switch off chiller and instrument
- please be aware that unless disassembled, this machine can
not be used for Western blot samples (contamination possible)
- little pieces of plastic or tissue can block the jet. Easy
to fix by taking off the chilled steel dome.
|
| Example
purification (Ni Sepharose FF) |
- 120L BAI cells gave 1 Kg of
wet cell pellet, frozen in LN2
- smash frozen pellet into small
pieces, be careful not to get pieces of plastic into suspension
- add 50 mM Tris pH 8 up to 2L,
20 EDTA-free PI tablets (Roche)
- stir until completely smooth
- run through cell disruptor at
20 KPSI, let foam settle for 10 min
- fill 12 type 19 rotor bottles
and balance (because of foam)
- run two rotors at 19.000 rpm
for 1 hour at 4°C
- decant supernatant and collect
- run supernatant at 20 ml/min
over 50 ml Amersham Ni Sepharose FF
- follow your usual elution protocol,
everything at 20 ml/min.
|
| JL 3/6/2005 |

