|
Dynamin 1 supplemental data
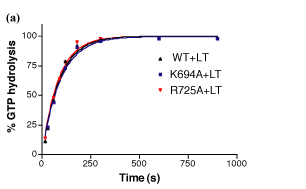
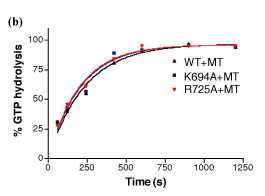
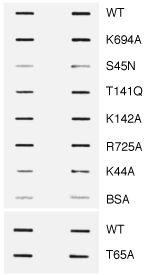
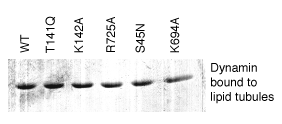
This material is supplemental to Marks et al., "GTPase activity of dynamin and resulting conformation change are essential for endocytosis." Nature 2001: 410, 231-235 (.pdf) Please click on one of the links belo w to view the data
|