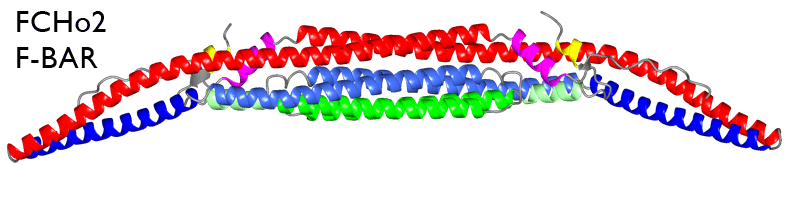
What is the F-BAR domain? a sub-family of the BAR superfamily of membrane curvature binding/bending proteins (see family members) (see movie of FCHo2 F-BAR domain -surface electrostatics).
How do we think F-BARs bind/bend membranes?
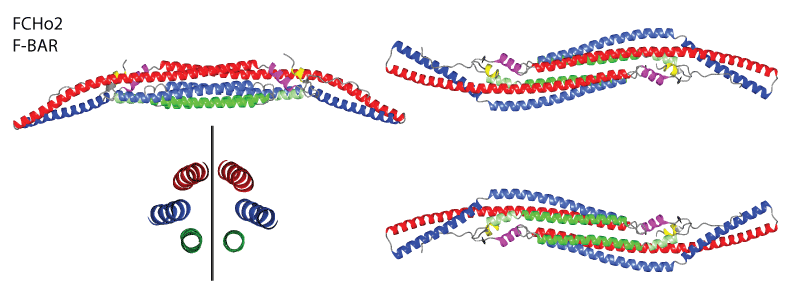
F-BAR domains can dimerise into the F-BAR module (see movie above). This module has an intrinsic crescent shape and binds to membranes via its concave face. The binding of many F-BAR modules causes the membrane to conform to this curvature. Although F-BARs have a N-terminal amphipathic helix like the N-BARs, this helix seems to only transiently interact with the membrane upon lipid binding, and may serve more toward helping dimerisation than curvature induction.
In vivo function of F-BAR proteins
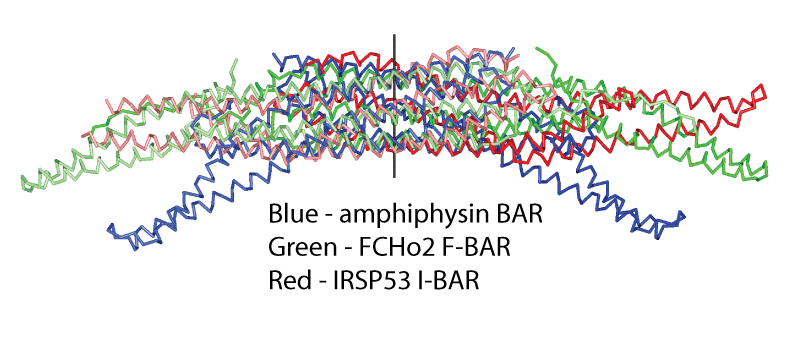
F-BAR domains are present in many proteins involved in membrane trafficking and frequently also linked to cytoskeletal dynamics. They are likely involved in the driving or sensing of extreme positive membrane curvatures (see in vitro tubulation showing 2 broad and narrow tubules). These curvatures are distinct from those achieved by BAR and N-BAR domains (see BAR domains and amphiphysin in vitro tubulation).