Amphipathic helices in Drosophila amphiphysin and other N-BAR proteins
This material is supplemental to Peter et al., "BAR Domains as Sensors of Membrane Curvature: The Amphiphysin BAR Structure" Science Express Nov 26 (abstract); print version Jan 23, 2004.
Fig. S2. Folding of an NH2-terminal amphipathic helix
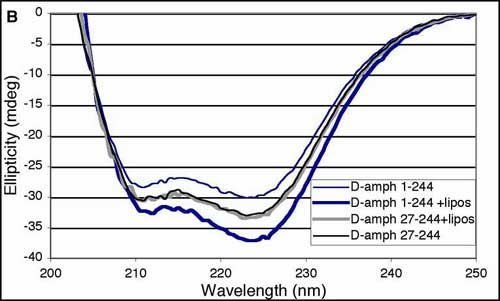
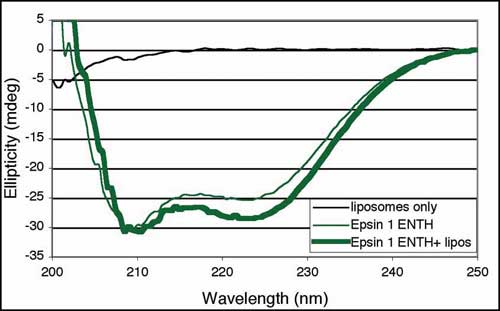
(A) Sequence alignment of predicted amphipathic helices in the the NH2-termini of proteins containing an N-BAR domain. Many other BAR-containing proteins do not contain this helix and thus this may help to define BAR domain subfamilies. Hydrophobic residues are highlighted in yellow, basic residues in blue, and acidic residues in red. Rvs161p, Rvs167p and Hob are yeast homologs of amphiphysin/endophilin. Abbreviations, d, Drosophila; r, rat; h, human; Cele, C. elegans; Sc, Saccharomyces cerevisiae; Spombe, Schizosaccharomyces pombe; m, mouse. (B) Top, Circular dichroism (CD) spectra of the N-BAR (residues 1-244) or BAR (residues 27-244) domain of Drosophila amphiphysin taken in the presence or absence of brain lipid liposomes. Minima at 208 and 222 nm indicate helical content; the deepening of the second minimum is typical of an increase in a-helix content. Similar results were obtained with rat amphiphysin1. Bottom, Control CD spectra showing the spectrum of liposomes only, and the effect of liposomes on Epsin1 ENTH (Epsin NH2-terminal homology domain), which folds an extra a-helix upon lipid binding (M. G. Ford et al., Nature 419, 361 (2002)).
The folding of the NH2-terminal amphipathic helix in amphiphysin could aid tubulation by direct interaction with the membrane, by protein-protein interactions between dimers, or both, but we cannot distinguish them at present. In solution, analytical ultracentrifugation showed no aggregation beyond a dimer, but higher oligomers were detected by crosslinking on liposomes (not shown). Mutation of phenylalanine 9 to glutamate in amphiphysin1 reduced but did not abolish tubulation (See Fig. 2E in main paper), in contrast to the effect seen in endophilin (K. Farsad et al., J. Cell Biol. 155, 193 (2001)). Mutation of lysine 15 (in Drosophila amphiphysin) or lysines 15 and 16 (in mammalian amphiphysin1) to glutamates had little effect.