Overexpression of BAR domains
This material is supplemental to Peter et al., "BAR Domains as Sensors of Membrane Curvature: The Amphiphysin BAR Structure" Science Express Nov 26 (abstract); print version Jan 23, 2004.
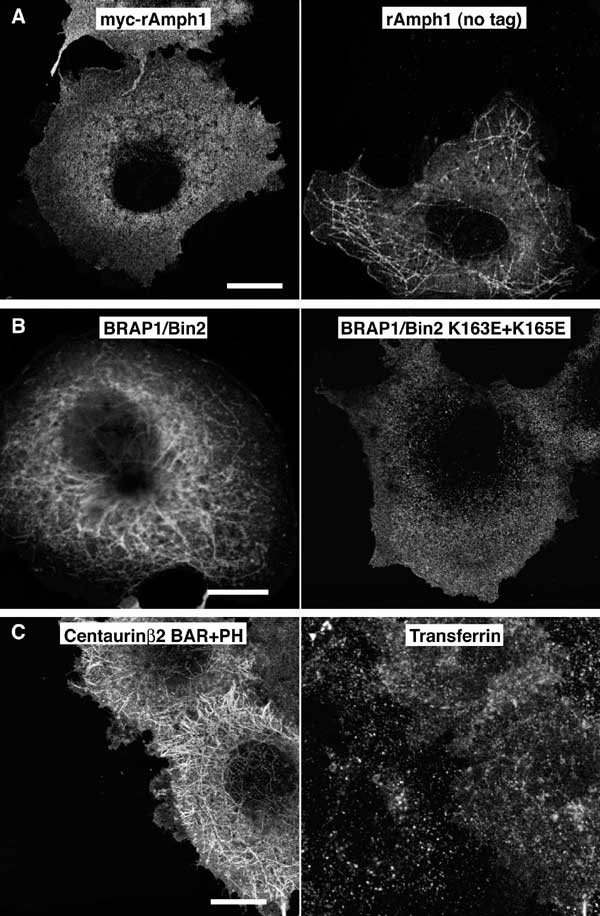
Fig. S3. Tubulation by BAR-containing proteins in vivo
COS-7 cells were transfected with pCMV vectors expressing NH2-terminally myc-tagged amphiphysin1 (Amph1), untagged amphiphysin1, BRAP1/Bin2 N-BAR, and centaurinBeta2 BAR+PH (CentaurinBeta2 is also known as ACAP2). Scale bar = 20mm. (A) Expression of NH2-terminally myc-tagged amphiphysin does not cause tubulation, while expression of the untagged protein does. (B) Expression of the wild-type N-BAR of BRAP1/Bin2 also causes tubulation, while the K163E+K165E mutant does not. (C) Overexpression of centaurinBeta2 BAR+PH causes extensive tubulation and a defect in transferrin trafficking. In many cases these tubules emanated from foci within the cell but we were not able to identify the compartment. However, the PH domain of centaurinBeta2 has been reported to bind PtdIns(3,5)P2 (Dowler et al., Biochem. J. 351, 19 (2000)) a lipid implicated in endosome to multivesicular body trafficking (Shaw et al., Traffic 4, 479 (2003)).
(click on image to see a higher resolution version (488kb) in a new window)