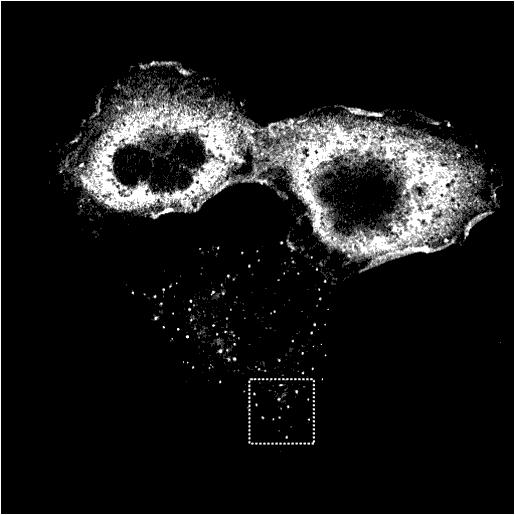
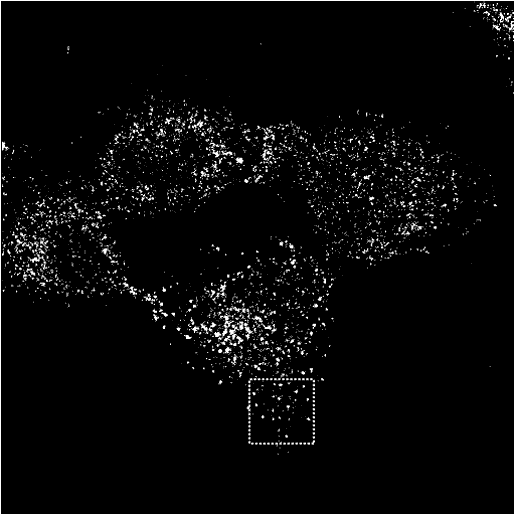
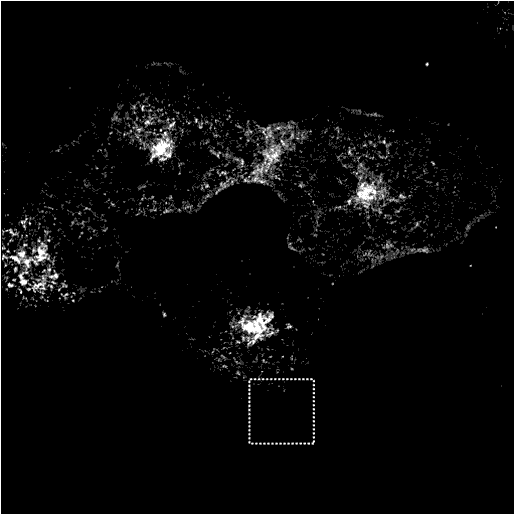
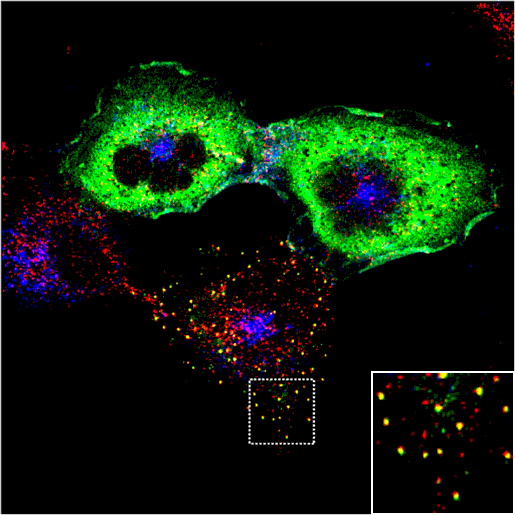
Immunofluorescence of wt and mutant Epsin 1 in COS cells
Summary of observations shown below:
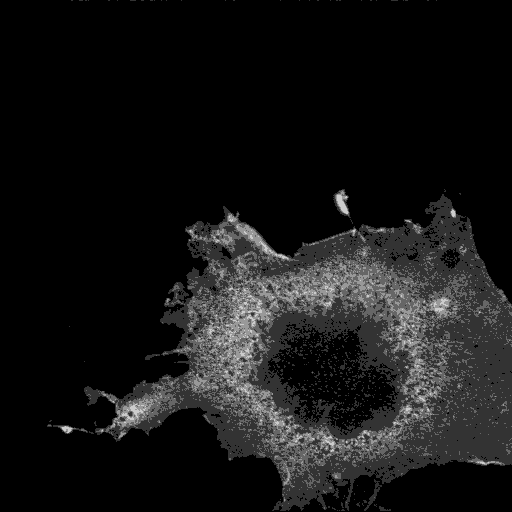
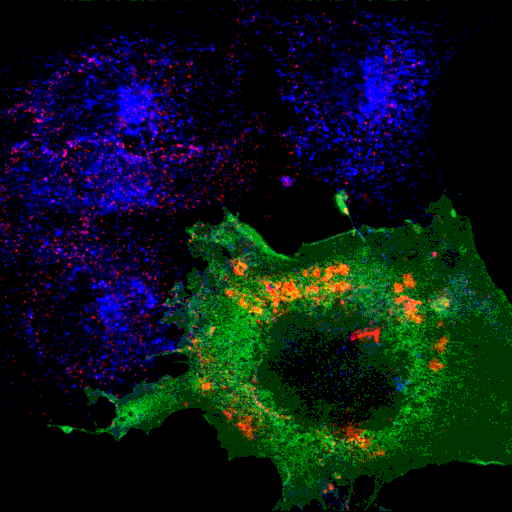
WT Epsin1 is cytoplasmic in some cell and punctate in others. Where it is punctate it colocalises with other endocytic markers (see other markers).
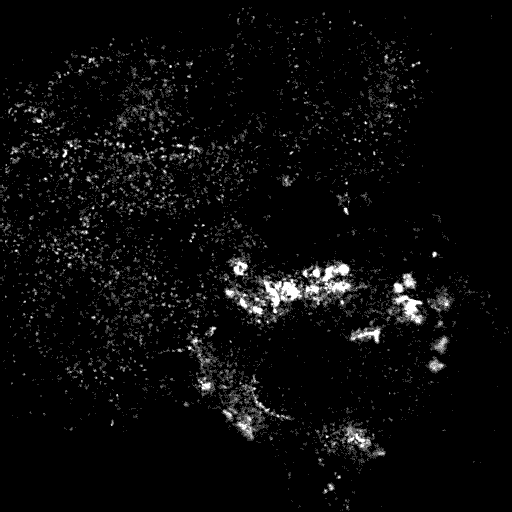
R63/R73 mutant does not bind PtdIns(4,5)P2 and so does not localise to puncta in any cells and in fact causes AP2 adaptors to become cytoplasmic. Thus this is a strong mutant of AP2-dependent endocytosis.
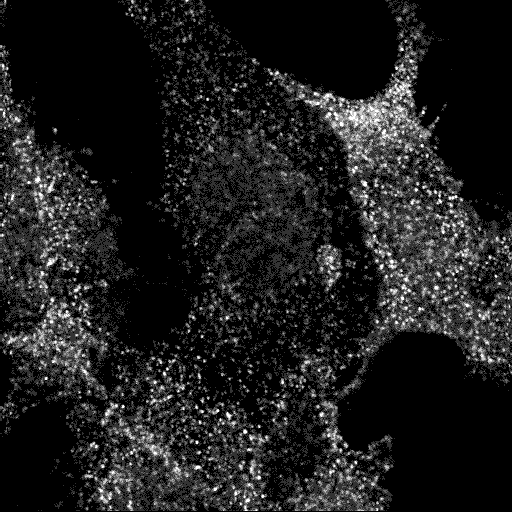
The ENTH domain of epsin1 is sufficient for membrane targeting. It localises to puncta and tubules, and mutants are cytoplasmic.
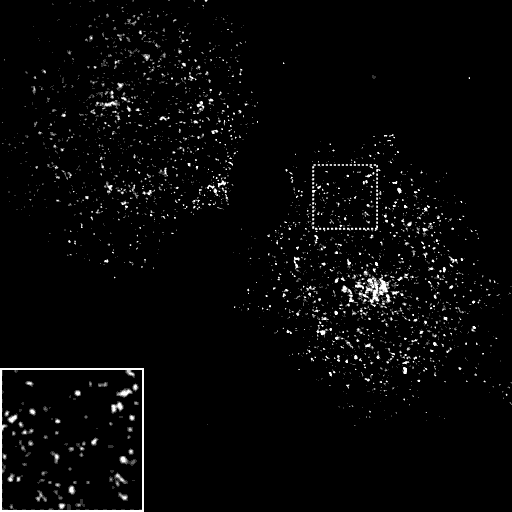
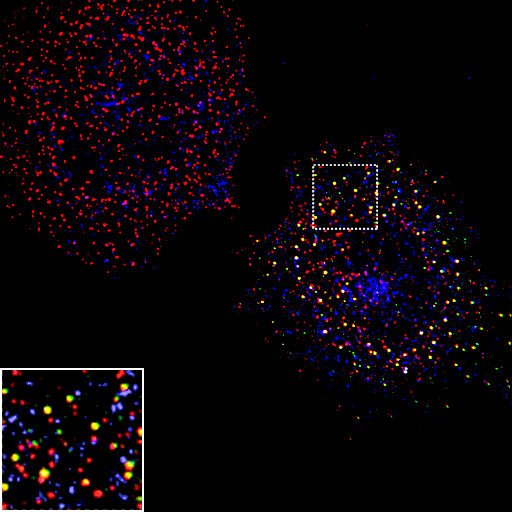
Helix zero mutants either can inhibit membrane association or promote association depending on whether they change the amphipathic nature of the helix. In vitro the L6Q mutant can associate weakly with membranes but helix zero does not insert. In the pictures below, this mutant does not prevent epsin targeting to clathrin-coated pits (because it does not disrupt PtdIns(4,5)P2 binding). However it does alter the kinetics of endocytosis.
This material is supplemental to Ford et al., "Curvature of clathrin coated pits driven by epsin" Nature, 419: 361-366 (.pdf). These are larger versions of the images shown in Figure 4.
Click on each image to enlarge it
 |
 |
 |
 |
|
wt Epsin1
|
AP2
|
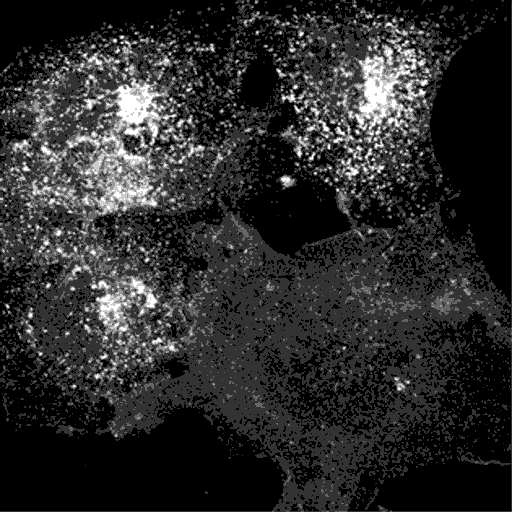
Transferrin
|
Merge
|
 |
 |
 |
 |
|
Epsin R63L H73L
|
AP2
|
Transferrin
|
Merge
|
 |
 |
 |
 |
|
Epsin ENTH domain
|
AP2
|
Transferrin
|
Merge
|
 |
 |
 |
 |
|
Epsin L6Q
|
AP2
|
Transferrin
|
Merge
|
Click here to see colocalization of Epsin with other endocytic markers