 |
|
|
|
In these pages we discuss the function of Epsin1 in clathrin-coated vesicle formation on PtdIns(4,5)P2 rich membrane zones
|
|
|
The domain structure is similar to AP180 and CALM but the proteins are functionally different (see domains). We crystallised the lipid binding domain, the Epsin N-Terminal Homology (ENTH) domain in the presence of the PtdIns(4,5)P2 headgroup (IP3)
|
|
|
 click image click image
|
Epsin 1 is similar to AP180 in that it has a PtdIns(4,5)P2 binding domain and can recruit clathrin to PtdIns(4,5)P2 containing membranes (see details of structure
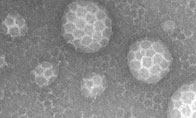
). However, unlike AP180, epsin can itself deform the lipid membrane (see EM), a process disrupted by point mutations. Epsin polymerises clathrin around these areas of invagination into patches that can be much larger than
the size of a coated vesicle (see gallery of images). We propose that epsin functions primarily at the edge of sites of invagination to actively drive the deformation of the membrane.
|
|
|
|
Functionally epsin1 co-localises with clathrin-coated pits predominantly on the plasma membrane in cells where one also finds an accumulation of other endocytic mark
ers (including AP2, EPS15 and dynamin II, see co-localisation gallery). The epsin1 PtdIns(4,5)P2 binding mutant R63/H73 mutant (see affinity measurements) is a strong inhibitor of AP2-depe
ndent endocytosis (see mutant gallery). This mutant that still binds to AP2 adaptors and thus drives the AP2s into the cytoplasm and out of clathrin-coated pits.
|
|
|
Further proposals:
A. Epsin1 is localised to the leading edge of a coated pit where the membrane is being actively bent, but it is not needed and indeed is not normally kept after the clathrin cage has stabilized the shape of the formaing pit.
B. Phosphorylation of helix 0 by a CCV kinase regulates membrane insertion and thus the initial budding.
C. It is interesting to speculate that epsin could form a break-point on the neck of a clathrin-coated vesicle given its perturbation of the bilayer and its ability to give a tighter curvature than dynamin or BAR domain protiens. Thus when dy
namin springs into action... the neck/cage boundary may be the weak point and therefore the break-point during vesicle scission.
C. EpsinR is a ubiquitous epsin1 homologue that functions in vesicle trafficking from the TGN and from endosomes (see EpsinR pages). It has a different lipid specificity and thus we propose that m
ost trafficking pathways will have an epsin-like molecule that can help drive membrane deformation.
Ford, M.G.J., Mills, I.G., Peter, B.J., Vallis, Y., Praefcke, G.J.K., Evans, P.R. and McMahon, H.T. (2002) Curvature of clathrin-coated pits driven by epsin. Nature 419: 361-366 (abstract). (.pdf) supplemental data
(see also News and Views and highlights in Nature Cell Biology, Nature reviews in molecular cell biology, and a mini review in Cell (Cell October 18, 2002: 111 (2):143-146)(summary)
|
|