Disruption of endocytosisWe have used 3 main methods to disrupt endocytosis in cells: use of inhibitory peptides, overexpression of protein domains and RNAi. Each method has advantages and disadvantages and thus it is preferable if a number of approaches can be used.
We first used the peptide approach in Marks et al 1998 where a dynamin peptide was made membrane permeable and used to inhibit synaptic vesicle recycling. This peptide is now available from Tocris. We are currently developing more potent tools.
|
 |
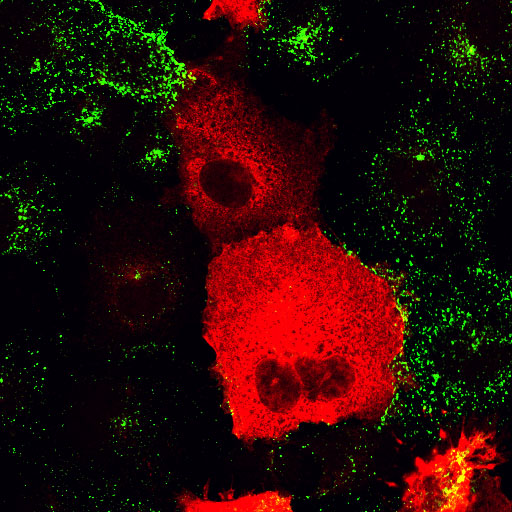 |
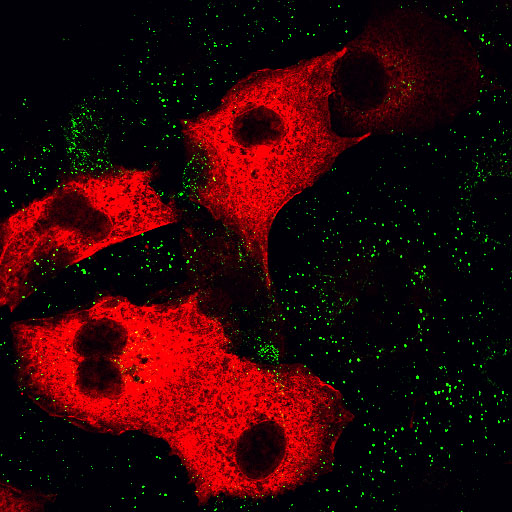
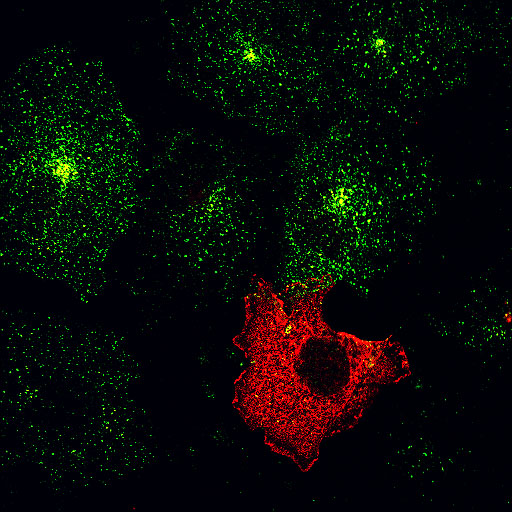
| AP180C-terminus/EGF | AP180C-terminus/Transferrin |
 |
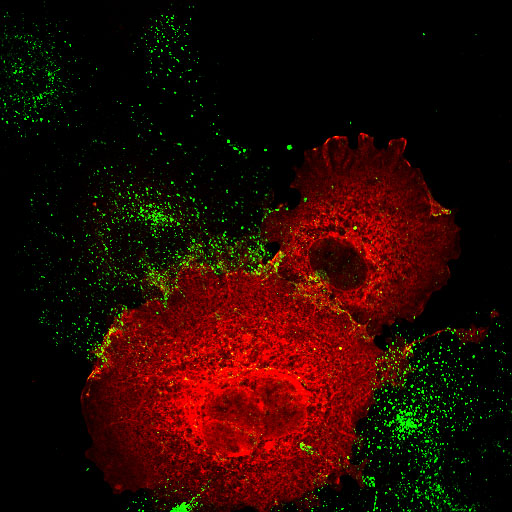 |
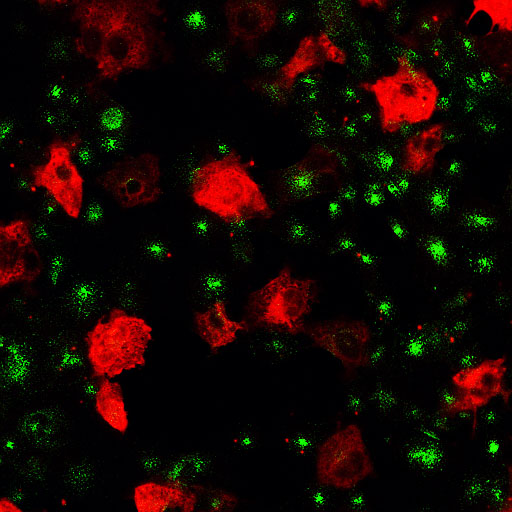
| Amphiphysin2 SH3 domain (low mag.)/Transferrin |
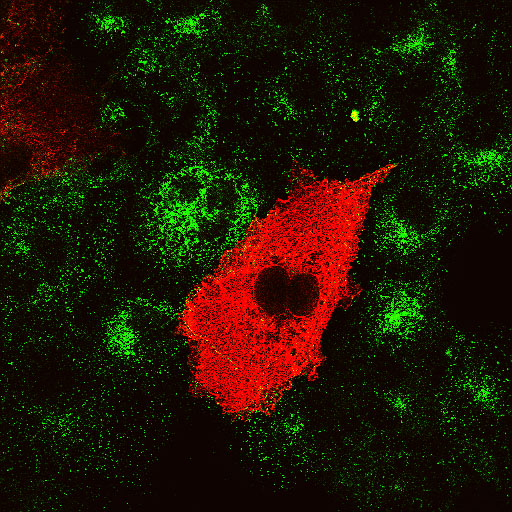
Amphiphysin2 SH3 domain Transferrin |
 |
 |
| Dyn PH domain mutant G532S,K535A Transferrin |
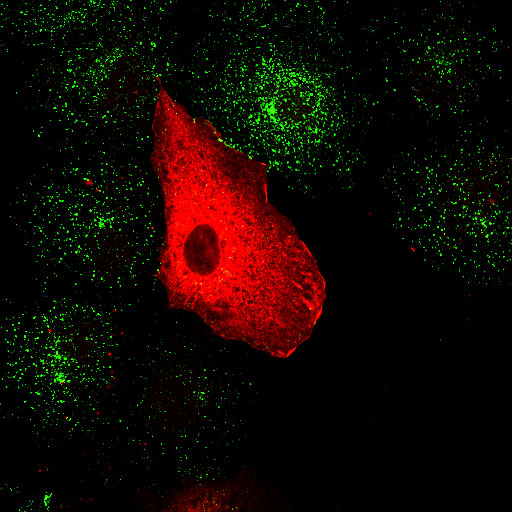
Alpha adaptin ear (EL1) Transferrin |
 |
 |
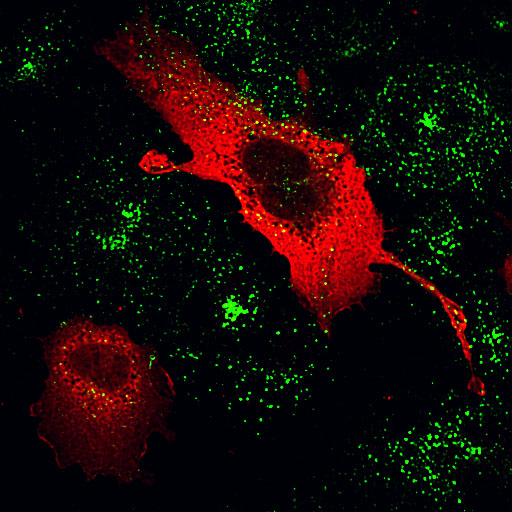
| Eps15 C-terminus/Transferrin | Dynamin PH domain mutant K535A/Transferrin |
 |
|
| AP2 adaptor Mu subunit EGF |
back to Techniques