|
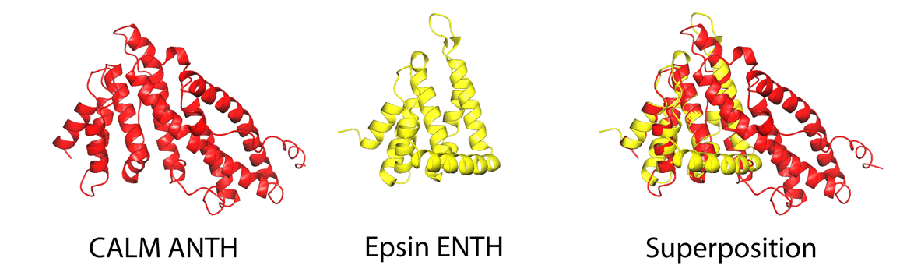
ANTH versus ENTH domains
AP180 N-Terminal Homology domain |
|
The AP180 family from yeast to man has a conserved N-terminal domain which we crystallised and for the first time showed that this domain is a PtdIns(4,5)P2 binding domain. There is greater than 50% amino acid identity in this domain between rat, C-elegans (UNC-11) and Drosophila (LAP).
|
|
|
| We solved the structure of AP180-2/CALM ANTH domain. The structure is a solenoid of 9 helices. The PtdIns(4,5)P2 binding residues are spread over several helices and has the conserved sequence Kx9Kx(K/R)(H/Y). An ANTH domain is also found in HIP1 and HIP1R and the PtdIns(4,5)P2 binding sequence is conserved. |
|
|
|
The ENTH domain is about half the size of an ANTH domain, but is also alpha-helical and in fact some of the helices superimpose very nicely (see below). The ENTH domain also binds to PtdInsPhosphates but using completely different residues. We have shown that the binding of PtdIns(4,5)P2 to epsin1 ENTH domain causes the folding of an additional N-terminal helix that can insert into the membrane and drive positive membrane curvature.
|
 |