Control of cell shape and the cytoskeleton during tube formation
We are using the formation of the salivary glands in the fly embryo as a model process to understand how cell shape changes are controlled by the cytoskeleton and interactions between cells in the epithelial sheet that is forming the glands.
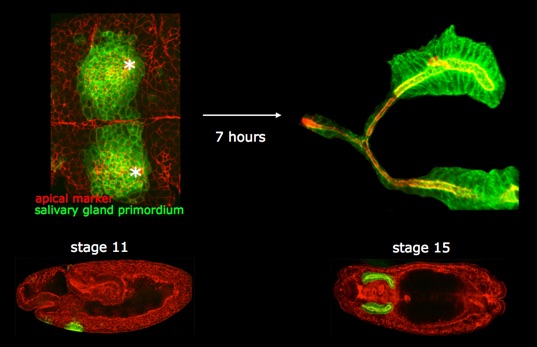
To do so, we analyse the dynamic behaviour of both actomyosin and microtubule cytoskeletons in live and fixed samples, and we trace the 3D shapes of cells fated to form the gland over time.
This analysis recently revealed that a supracellular actomyosin cable forms around the salivary gland placode and exerts centripetal force that assists tube invagination.
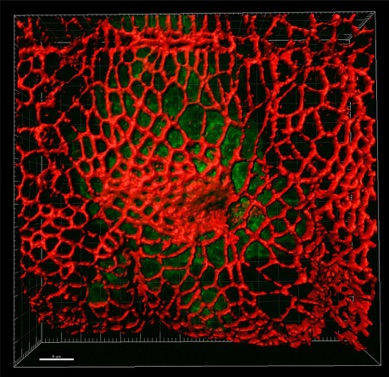
Actomyosin function during tube formation.
Using the excellent genetic tools available in Drosophila we image and dissect the live behaviour of cytoskeletal components during tissue formation. We focus particularly on the role of non-muscle myosin II during the early stages of tissue bending and invagination in the embryonic epidermis.
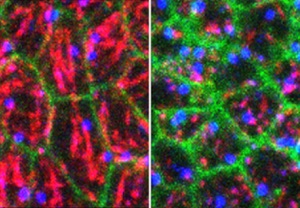
Function of the microtubule cytoskeleton during tube formation.
In contrast to actomyosin for which several roles have been defined during morphogenetic processes, the role of the microtubule cytoskeleton during tissue formation is much less clear. We analyse the role of microtubules during tube formation using advanced imaging methods, using genetic tools to disrupt microtubules in specific cells only.
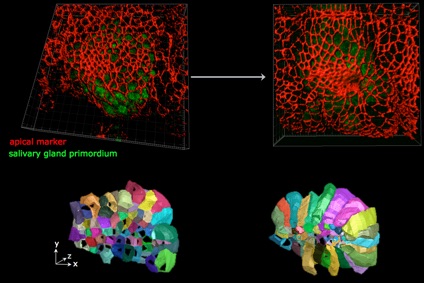
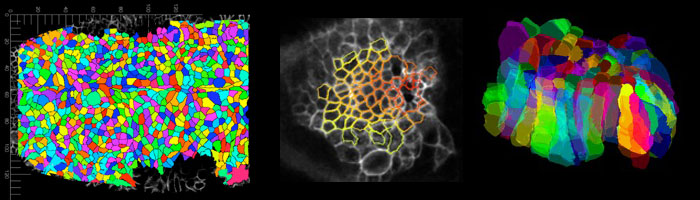
Cell behaviours during 3D tube formation.
Much analysis of morphogenesis is focussing on events that take place within the apical junctional domain of epithelial cell, where actomyosin and adherens junctions are located and exert their effects. The apical domain of many epithelial cells constitutes only a small proportion of the total cell. We trace and analyse cell shape changes in 3D over time (in a collaboration with Guy Blanchard and Richard Adams at the University fof Cambridge), to identify signature cell behaviours in 4D that drive tube formation and tissue bending.
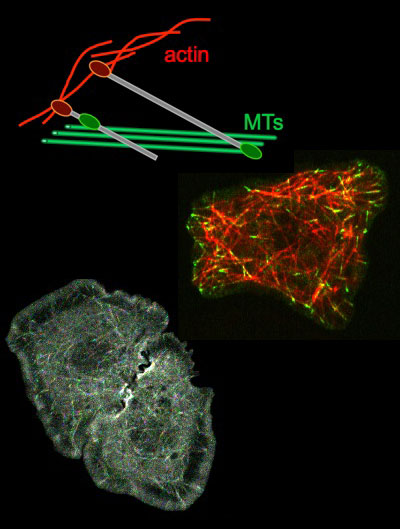
Analysis of cytolinker function in determining cell shapes.
Cytoskeletal crosslinkers or cytolinkers are proteins with the ability to interact with several cytoskeletal systems at he same time. This allows them to help coordinate the behaviour of different cytoskeletal systems.
The spectraplakin family of cytolinkers, represented by the protein Short Stop (Shot) in the fly, fulfil many important functions in a variety of tissues during development. We currently investigate Shot’s role in the embryonic epidermis.
The cytolinker Pigs, like Shot containing an actin-binding CH and a microtubule-binding Gas2 domain, is important during egg chamber morphogenesis in the fly ovary. Here, Pigs is a downstream target of Notch signalling and downregulates Notch signalling itself in a negative feed-back loop. We currently investigate Pigs’ molecular role through structure-function analysis.
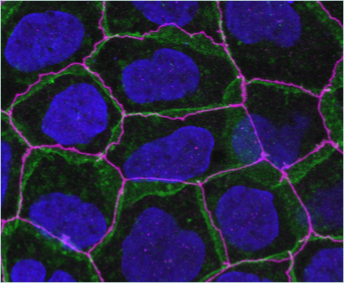
How do adhesion or signaling receptors affect the cytoskeleton?
We use mammalian epithelial culture systems to address how surface receptors, in particular those not classically implicated in cell-cell interactions might affect epithelial integrity and junctional cytoskeleton. For this we use both Caco2 cell, a human colon carcinoma cell line, as well as primary bronchial epithelial cells grown at the air-liquid interface.
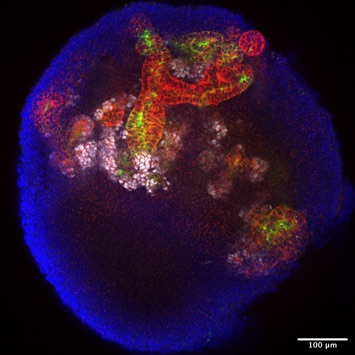
MET or the establishment of epithelial polarity and tubulogenesis in human renal organoids
In a new adventure, we have started to use iPSC-derived human renal organoids as a model system to understand how at the beginning of kidney tubule formation epithelial polarity is established in the renal vesicle. We also want to understand the mechanisms that then drive the growth, expansion and functional segmentation of the tube of the human nephron.