Research
Introduction. Improving oligonucleotide delivery using Cell-Penetrating Peptides
For many years, projects in the Gait group have revolved around synthetic oligonucleotides and their analogues especially with an emphasis on development of enabling chemical synthesis methods. Increasingly, applications have shifted from those in molecular biology towards applications in molecular medicine, as oligonucleotide analogue synthesis has become more synthetically accessible. Key for success has been obtaining good cell and in vivo delivery of oligonucleotides, something that was hard to achieve in the 1980s and 1990s in biotech and pharma industry. Building on a collaboration forged with the laboratory of Bernard Lebleu in Montpellier starting in 1996, our group has been one of several, mostly in Europe, to pioneer the use of cell-penetrating peptides (CPPs) as covalently conjugated delivery agents for oligonucleotides. CPPs are usually short (10 -30 residue) peptides that are commonly full of cationic residues (such as Lys, Arg and His) as well as some hydrophobic residues, and such peptides were eventually shown to be taken up by cells mostly through endosomal delivery pathways, especially at lower concentrations. Between 1996 and 2005, we developed methods of conjugation of CPPs with oligonucleotides. However, conjugation of CPPs with negatively charged oligonucleotides, although resulting in improved delivery into endosomes, did not lead to efficient translocation into cytosol and nucleus and thus only limited biological activity was achieved (Figure 1).
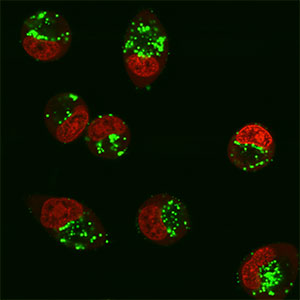
FIGURE 1 Delivery using an R4-Tat CPP disulfide linked to a 12-mer anti-TAR 7xOMe/5xLNA-FAM-labelled oligonucleotide at 2.5 µM, 5 h incubation in HeLa cells with nuclei stained red, showing cystolic location of conjugates (2005 Arzumanov et al).
In 2005, we found a substantial improvement in cell delivery and activity when we switched to the use of charge-neutral oligonucleotide analogues, the first of which was peptide nucleic acids (PNA) [1]. This led to new projects in use of peptide-PNA (P-PNA) conjugates targeting pre-mRNA in the cell nucleus to redirect splicing. Further studies initiated in 2007 in collaboration with the Wood group in Oxford led to studies on exon skipping of the dystrophin gene in a mouse model of Duchenne muscular dystrophy, first using P-PNA and then more recently switching to a second charge neutral oligonucleotide type, called phosphoramidate morpholino oligonucleotides (PMO). PNA continued to be used in group projects for targeting microRNA, where rules of good cell delivery were identified in model systems using hepatocytes [3] as well as in B cells [4]. In 2014 the Gait group consolidated effort to concentrate on projects to develop P-PMO and P-PNA for applications in neuromuscular diseases in collaboration with Matthew Wood and colleagues in Oxford.
[1] Cell-penetrating peptide conjugates of peptide nucleic acids (PNA) as inhibitors of HIV-1 Tat-dependent trans-activation in cells”. J.J. Turner, G. Ivanova, B. Verbeure, D. Williams, A.A. Arzumanov, S. Abes, B. Lebleu and M. J. Gait. Nucl. Acids Res. (2005) 33, 6837-6849).
[2] Efficient splicing correction by PNA conjugation to an R6-Penetratin delivery peptide. S. Abes, J. J. Turner, G. D. Ivanova, D. Owen, D. Williams, P. Clair, M. J. Gait and B. Lebleu, Nucleic Acids Res. (2007) 35, 4495-4502).
[3] miR-122 targeting with LNA/2’-O-methyl oligonucleotide mixmers, PNA and PNA-peptide conjugates, M. Fabani and M.J. Gait, RNA, (2008) 14, 336-346).
[4] Efficient inhibition of miR-155 with peptide nucleic acids in primary B-cells and in vivo. M.M. Fabani, C. Abreu-Goodger, D. Williams, P.A. Lyons, A. Torres, K.G.C. Smith, A. Enright, M.J. Gait and E.Vigorito, Nucleic Acids Res. (2010) 38, 4466-4475).
Duchenne Muscular Dystrophy (DMD)
Duchenne muscular dystrophy (DMD) is a severe muscle-wasting disease in young boys caused by deletions or point mutations in the pre-mRNA for dystrophin that result in out-of-frame transcripts and hence a non-functional truncated dystrophin protein. Synthetic splice-switching oligonucleotides (SSOs) have been developed as new treatments for DMD whereby an SSO is targeted to the pre-mRNA and mediates splicing redirection to restore the reading frame of the dystrophin gene via exon skipping, and thus to generate a shorter but functional dystrophin protein isoform. Of the several SSO chemistries developed for exon skipping in DMD and other neuromuscular diseases only two have been used in clinical trials so far, namely 2’-O-methyl phosphorothioates (2’-OMe/PS) and PMO [5].
PMOs conjugated to CPPs (P-PMOs) have been designed in order to enhance delivery into cells, the most effective of which are Arg-rich. Significantly improved uptake in an mdx mouse model of DMD, where the dystrophin gene has a point mutation in exon 23, is seen both in muscle cell culture and in skeletal muscle following systemic delivery. Starting from a derivative of the already known CPP Penetratin containing 6 additional arginine residues (R6-Pen), we derived a series of PNA/PMO internalization peptides (Pips) with improved stability to serum proteolysis, which are comprised of a hydrophobic core region flanked on each side by an Arg-rich domain containing aminohexanoic (X) and β-alanine (B) spacers. Two of these Pip peptides, Pip2a and Pip2b, when conjugated to a dystrophin exon 23-specific peptide nucleic acid (PNA) SSO, showed strong exon skipping and dystrophin production in mdx muscle cells and following intramuscular injection into the tibialis anterior (TA) muscle of the mdx mouse [6]. Later, Pip5 versions as PMO conjugates showed remarkably high dystrophin production in heart in addition to skeletal muscle, following systemic delivery into mdx mice (Figure 2) [7].
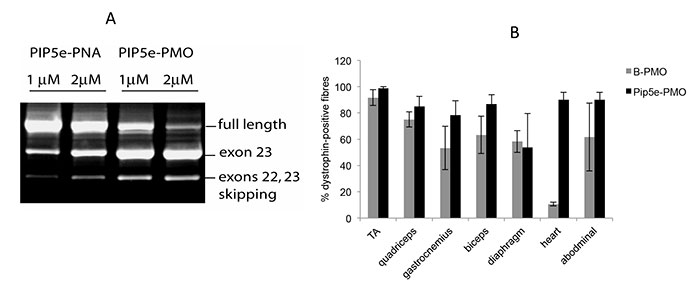 FIGURE 2. A. A typical exon-skipping assay in mouse mdx cells showing that P-PMO is more active than P-PNA. B. In vivo assay in mdx mice showing that Pip5e-PMO has considerably higher exon skipping activity in heart muscle. |
In a further Pip6 series of peptides, the hydrophobic core was found to be essential for dystrophin production in heart, but the precise sequence was found not to be important [8]. Pip6a-PMO in particular has become a paradigm for mouse physiology studies in DMD, together with collaborators from Oxford and London [9,10] as well as understanding of the muscle cell uptake and trafficking, with collaborators in Montpellier [11]. Shorter Pip7 to Pip9 variants were designed in order to provide leads for potential clinical use. Pip peptides have been included in two patent applications that have been issued in the USA and licensed to pharmaceutical companies for exploitation of their delivery potential of PMO for various diseases.
Meanwhile, further variants of these Arg-rich CPPs, called D-PEP, have also been developed in several families as conjugates of PMO. These also contain fewer Arg residues than Pip6a, which in work funded by the French muscular dystrophy society AFM has been shown to be too toxic in mice to be considered as a starting point for drug development (unpublished studies). Work is under way currently to choose lead D-PEP variants as clinical leads. In addition the D-PEP IP forms the basis of a platform technology to be licensed to a new MRC-Oxford University spin-off company to take a P-PMO to a clinical trial. As this research group closes in 2017, the peptide development work will be continued at the University of Oxford and hopefully also in the spin-off company.
[5] A chemical view of oligonucleotides for exon skipping and related drug applications. P. Järver, L. O’Donovan and M.J. Gait Nucleic Acids Therapeutics (2014) 24, 37-47.
[6] Improved cell-penetrating peptide-PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. G.D. Ivanova, A.Arzumanov, R. Abes, H. Yin, M.J.A. Wood, B. Lebleu and M.J.Gait, Nucleic Acids Res. (2008), 36, 6418-6428.
[7] Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. H. Yin, A, F. Saleh, C. Betts, P. Camiletti, Y. Seow, S. Ashraf, A. Arzumanov, M. J. Gait and M.J.A. Wood, Molecular Therapy (2011) 19, 1295-1303.
[8] Pip6-PMO, a new generation of peptide-oligonucleotide conjugates with improved cardiac exon skipping activity for Duchenne muscular dystrophy treatment. C. Betts, A.F. Saleh, A.A. Arzumanov, S.M. Hammond, C. Godfrey, T. Coursindel, M.J. Gait and M.J.A.Wood, Molecular Therapy Nucleic Acids (2012) 1, e38.
[9] How much dystrophin is enough: defining dystrophin levels for clinical efficacy in DMD. C. Godfrey, S. Muses, G. McClorey, K. Wells, T. Coursindel, R. Terry, C. Betts, S. Hammond, L. O'Donovan, J. Hildyard, S. EL Andaloussi, M.J. Gait, M.J.A. Wood and D.J. Wells, Human Mol. Gen. (2015) 24, 4225-4237.
[10] Prevention of exercised induced cardiomyopathy following Pip-PMO treatment in dystrophic mdx mice. C.A. Betts, A.F. Saleh, C.A. Carr, S.M. Hammond, A.M.L. Coenen-Stass, C. Godfrey, G. McClorey, M.A. Varela, T.C. Roberts, K. Clarke, M.J. Gait and M.J.A. Wood, Scientific Reports, (2015) 5:8986.
[11] Cellular trafficking determines the exon skipping activity of Pip6a-PMOin mdx skeletal and cardiac muscle cells. T. Lehto, A. Castillo Alvaraz, S. Gauck, M.J. Gait, T. Coursindel, M.J.A. Wood, B. Lebleu and P. Boisguerin, Nucleic Acids Res. (2014) 42, 3207-3217.
Enabling Peptide Chemistry Methods
Several new enabling chemical methods have been developed utilising the power of peptide chemistry to further enhance the utility of use of CPPs as conjugates of PNA and PMO. These include:
1. A SELPEPCON method of parallel synthesis of peptide conjugates of bio-cargoes on scale suitable for cell screening as exemplified for PNA [12] and recently also for PMO [13].
[12] Parallel synthesis and splicing redirection activity of cell-penetrating peptide conjugate libraries of a PNA cargo. P.J. Deuss, A.A Arzumanov, D.L. Williams and M.J. Gait Org. Biomol. Chem. (2013) 11, 7621-7630.
[13] Parallel synthesis of cell-penetrating peptide conjugates of PMO towards exon skipping enhancement in Duchenne muscular dystrophy. L. O’Donovan, I. Okamoto, A. A. Arzumanov. D.L. Williams. P. Deuss and M.J. Gait. Nucleic Acids Therapeutics (2014) doi: 10.1089/nat.2014.0512.
2. A method for fluorescent labelling of P-PMO [14],
[14] Development of a general methodology for labelling peptide conjugates of morpholino (PMO) oligonucleotides using alkyne-azide click chemistry. F. Shabanpoor and M.J. Gait, Chem. Commun. (2013) 49, 10260-10262.
3. A method of conjugating two PMOs targeted to two different genes to a single CPP [15],
[15]. Bi-specific splice-switching PMO oligonucleotides conjugated via a single peptide active in a mouse model of Duchenne muscular dystrophy. F. Shabanpoor, G. McClorey, P. Järver, A. Saleh, M.J.A. Wood and M.J. Gait, Nucleic Acids Res. (2015) 43, 29-39.
Spinal Muscular Atrophy (SMA)
In a grant funded by the MRC DPFS/DCS programme (Wood, Muntoni, Gait), we began in 2014 on a second neuromuscular disease Spinal muscular atrophy (SMA), a leading genetic cause of mortality in young children. SMA is primarily due to lower motor neuron degeneration and progressive muscle weakness resulting from the loss of the ubiquitous survival motor neuron 1 gene (SMN1). Humans have a second nearly identical copy, SMN2, which differs from SMN1 by a single nucleotide transition within exon 7 leading to the predominant generation of an alternative exon 7-excluded transcript and only marginally functional protein. SMN2 therefore fails to compensate for loss of SMN1 unless sufficient copies are present to generate functional levels of full-length SMN protein. We have used antisense splice-switching oligonucleotides to enhance SMN2 pre-mRNA exon 7 inclusion via steric block of splice regulatory pre-mRNA elements. Targeting the intron splice silencer N1 (ISS-N1) site within intron 7, by deletion or SSO-mediated splice switching, improves exon 7 inclusion. ISSN1–targeted SSOs used to treat pre-symptomatic severely affected neonatal SMA mice, via systemic or intra-cerebroventricular administration, extend survival from 10 to >100 d, although SSO targeting to the CNS is also essential, since there is also evidence for a peripheral role for the SMN in SMA.
We have recently shown that Pip6a-PMO targeted to ISSN1 and delivered by intravenous injection yields SMN expression at high efficiency in peripheral and CNS tissues, resulting in profound phenotypic correction at doses an order-of-magnitude lower than required by standard naked SSOs [16]. Survival is dramatically extended from 12 d to a mean of 456 d, with improvement in neuromuscular junction morphology. In addition up-regulation of SMN2 transcripts were seen in all parts of the brain and spinal cord in an adult SMA mouse model, suggesting that the Pip6a-PMO crosses the blood-brain barrier. Similar increases in SMN2 transcript levels in the brain and spinal cord in the adult mouse model were also seen for a branched ApoE derivative peptide conjugated to an ISSN1-targeting PMO [17].
This work has been extended to the development of further peptides that ahave the same brain and spinal cord delivery properties for attached PMO whilst showing greater tolerability in mice than Pip6a. Here a D-PEP5 peptide family is showing particularly high promise for such delivery into the adult SMA mouse model whilst showing considerably shorter mouse recovery times. It is hoped that the D-PEP5 peptides will also form part of the IP basis for the founding of the MRC-Oxford University spin-off company.
[16] Systemic peptide-mediated, oligonucleotide therapy improves long-term survival in spinal muscular atrophy. S. M. Hammond, G. Hazell, F. Shabanpoor, A. F. Saleh, M. Bowerman, J. N. Sleigh, K. M. Meijboom, H. Zhou, F. Muntoni, K. Talbot, M. J. Gait and M. J. A. Wood, Proc. Natl. Acad. Sci. USA (2016) 113, 10962-10967.
[17] Identification of a peptide for systemic brain delivery of a morpholino
oligonucleotide in mouse models of spinal muscular atrophy. F. Shabanpoor, S. M. Hammond, F. Abendroth, G. Hazell, M.J.A. Wood and M.J. Gait Nucleic Acids Therapeutics (2017) 27, 130-143.
Myotonic dystrophy
In a grant funded 2014-2017 by the French muscular dystrophy association AFM (Wood, Gait, Puymirat and others), we have focussed on myotonic dystrophy type 1 (DM1), which is a neuromuscular disease characterized by myotonia, distal muscle weakness, heart conduction defects, and, in the congenital form, a delay in myogenesis and severe cognitive abnormalities. DM1 is caused by the presence of abnormal expanded CTG repeats within the 3′ untranslated region of the DMPK gene (2). We have targeted the expanded CUG repeats in the 3’-region of the DMPK pre-mRNA using Pip6a-PMO, whilst also in parallel investigating LNA/DNA phosphorothioate gapmer oligonucleotides for targeting the coding region of the DMPK gene in DM1 disease. Preliminary results have suggested that in a DM1 mouse model myotonia can be reduced by systemic injections of Pip6a-PMO. Extension work is now underway to apply improved D-PEP-PMO conjugates for targeting the DMPK toxic repeats towards the development of a clinical candidate for treatment of DM1.
Mitochondrial diseases
In a 3-way collaboration with Bob Lightowlers (University of Newcastle) and Mike Murphy (MRC Mitochondrial Biology Unit, Cambridge), we have investigated the potential for peptide delivery of a PNA cargo across the mitochondrial membrane barriers as a potential treatment for targeting mutations in the mitochondrial genome that give rise to mitochondrial genetic diseases. We used a mitochondria-targeted cyclooctyne (MitoOct) that accumulates several hundred fold in the mitochondrial matrix, driven by the membrane potential. There, MitoOct reacts through click chemistry with an azide on the target molecule to form a diagnostic product that can be quantified by mass spectrometry. Because the membrane potential-dependent MitoOct concentration in the matrix is essential for conjugation, we can now determine definitively whether a putative mitochondrion-targeted molecule reaches the matrix. We have now showed that a conjugate of a short PNA oligonucleotide with the leader sequence of a mitochondrially delivered protein reaches the mitochondrial matrix [18, 19]. This “ClickIn” approach will facilitate development of mitochondrially targeted oligonucleotide therapies. The chemistry of peptide-PNA conjugates will be continued by Kurt Hoogewijs at the University of Gent, Belgium, in conjunction with Bob Lightowlers and Mike Murphy.
[18] Assessing the delivery of mitochondria-targeted molecules using Click chemistry. K. Hoogewijs, A.M. James R.A.J. Smith, M.J. Gait, M.P. Murphy and R. Lightowlers, ChemBioChem. (2016) 17, 1312-1316.
[19] ClickIn: a flexible protocol for quantifying mitochondrial uptake of nucleobase derivatives. K. Hoogewijs, A.M. James, R.A.J. Smith, F. Abendroth, M.J. Gait, M.P. Murphy and R.N. Lightowlers, Interface Focus (2017) 7, 20160117.