The Central Sorting System of The Cell.
About The Golgi
In this page you will learn about the complexity and importance of the Golgi apparatus, and what aspects are being studied by the Munro lab.
The Golgi apparatus , also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destinations. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.
Sean Munro and his lab primarily work on the Golgi apparatus. Their work focuses on 2 questions. 1) How do the glycosylation enzymes stay in the Golgi whilst the secreted proteins move past them? 2) How are the transport vesicles that are destined for the Golgi specifically captured so they can deliver their ‘cargo’ to the correct part of the Golgi?
Rabs and Arfs – the regulators of Golgi traffic.
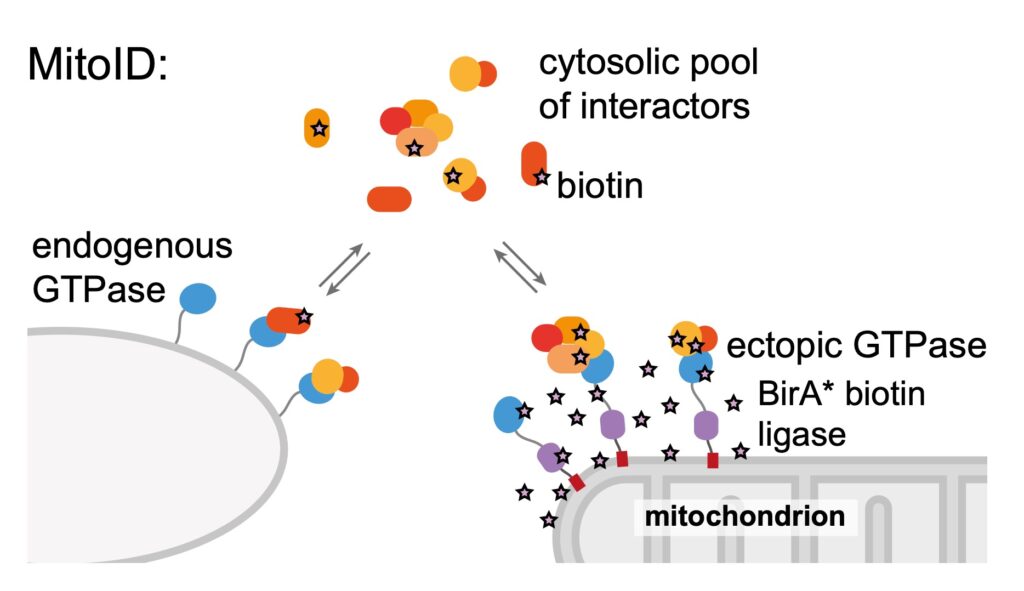
Many proteins stick to specific organelles by binding to small GTPases of the Rab and Arf families. For example, COPI binds to Arfs, and amongst the golgins, GM130 binds to Rab1, TMF binds to Rab6 and golgin-97 and golgin-245 bind to Arl1. We examine how exchange factors activate specific Rabs and Arfs only the Golgi so as to ensure the specificity of membrane traffic. To identify new proteins that bind to Rabs we have developed a very efficient in vivo method called MitoID and are investigating new interactors for Rab1.
How do the resident enzymes of the Golgi stay in place?
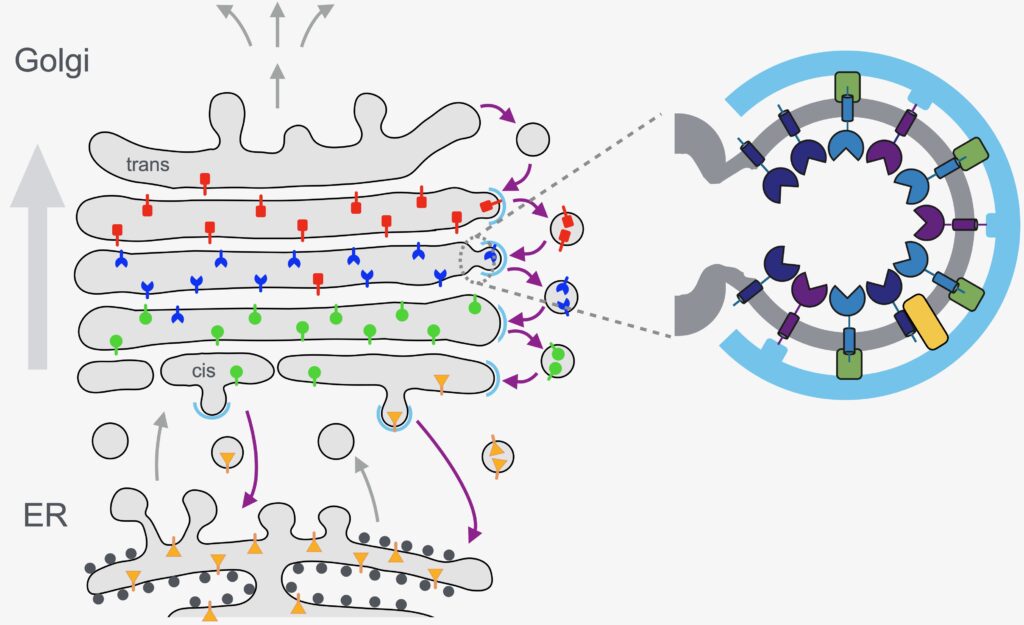
The Golgi is home to a host of enzymes that remain in the organelle whilst newly made proteins and lipids proteins continuously arrive from the ER and then depart to destinations elsewhere in the cell. Many of these resident enzymes are glycosyltransferases that act on glycoproteins and glycolipids, whilst others import and export activated sugars or ions, or are proteases or components of membrane traffic. It is now widely believed that these residents are continuously recycled in COPI-coated vesicles that bud of cisternae and then fuse to earlier cisternae as the stack progressively matures. We study how different residents are selectively included into COPI-coated vesicles in different parts of the stack. We have characterised the cytosolic protein GOLPH3 as an adaptor that recruits numerous enzymes in to vesicles, and are now testing further candidates for specific roles in this continuous carousel of cisternal maturation and vesicular regression.
Golgins and the capture of transport vesicles.
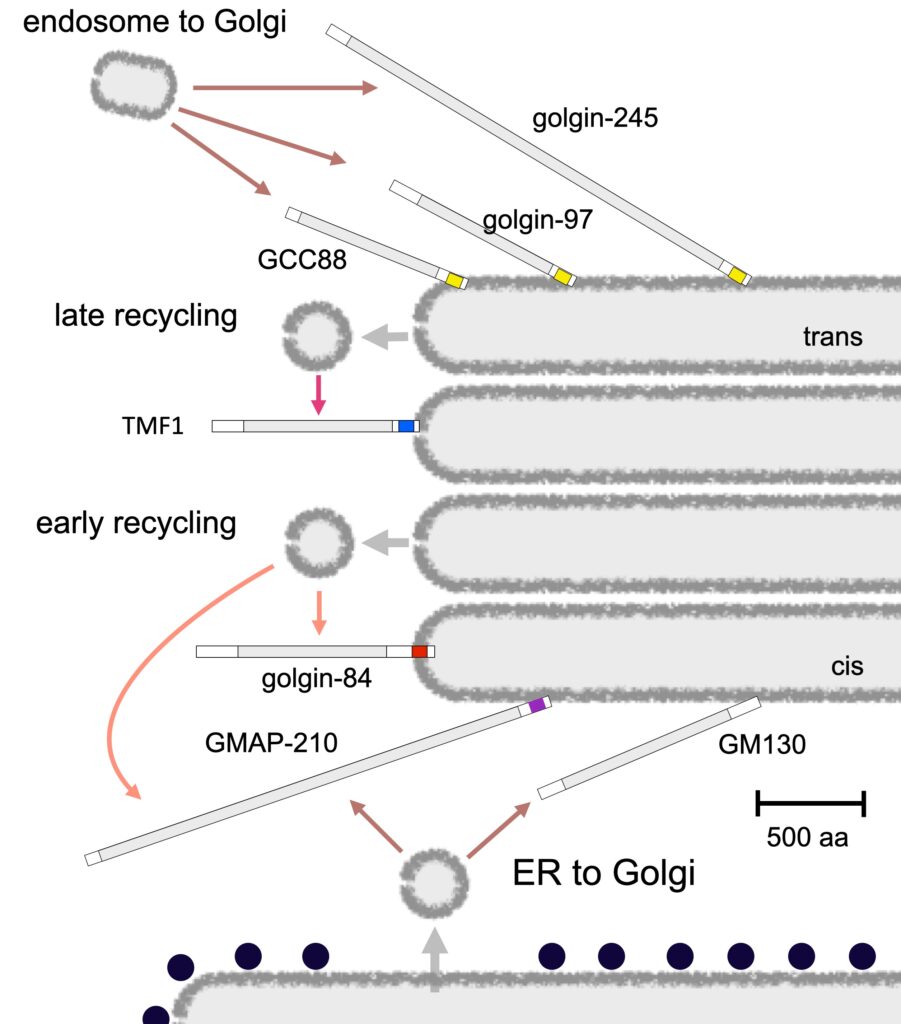
Transport vesicles arrive at the Golgi to deliver newly-made proteins from the ER, or proteins recycling from endosomes, or Golgi residents recycling within the Golgi stack. One fundamental question in membrane traffic is how the different types of transport vesicle only fuse with the correct destination organelle. We have shown that the vesicles arriving at the Golgi are captured by the golgins, long coiled-coil proteins that project out into the cytoplasm. Different golgins are present on different parts of the stack, and each captures a specific type of transport vesicle. We would like to understand how they can recognise specific vesicles. We have found that two golgins capture vesicles coming from endosomes via a linker protein call TBC1D23, and are investigating how it works and looking for the mechanism used by other golgins by studying both mammalian tissue cultures and Drosophila.
The Unknome Of The Golgi Apparatus
Sean Munro and the other members of the Golgi research team are performing extensive experimental work on the proteins that are found in the mysterious Golgi apparatus. These proteins are essential for life, found in many organisms and have been overlooked in prior Golgi research. Updates will be issued and found first on this site, when further information is found by the research team.