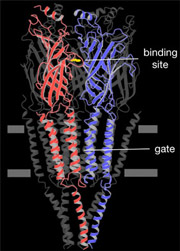
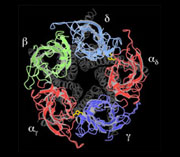
An atomic model of the closed channel, obtained from a 4Å density map, finally revealed the intricate folding of the αγ, β, δ, αδ and γ polypeptide chains. Each subunit is a long rod divided into three distinct parts: an N-terminal extracellular part organised around a β-sandwich core, a membrane-spanning part composed of four α-helices and an intracellular part containing one α-helix.
Five α-helices, one from each subunit, encircle the central membrane pore. These helices come together symmetrically near the middle of the membrane, forming a constricting hydrophobic girdle that is about 8Å long and 6Å across. The hydrophobic region contains no polar groups that could substitute for the hydration shell of the ion and so the ion is, in effect, too big to pass through. Hence the gate works as an energy barrier, rather than a physical barrier, in preventing ion permeation across the membrane.
Key publications:
Miyazawa, A., Fujiyoshi, Y. and Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949-955 (2003). (pdf)
Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J. Mol. Biol. 346, 967-989 (2005). (pdf)