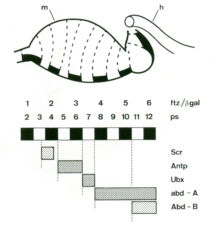
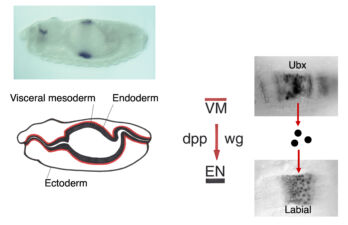
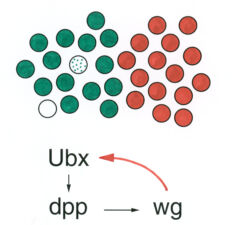
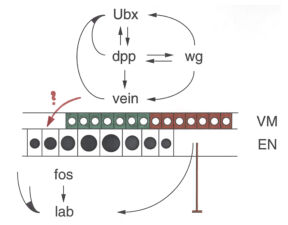
Early on, we discovered a discrete midgut-specific enhancer upstream of the Ultrabithorax promoter whose control was distinct from that of the epidermis-specific enhancers and silencers. This enhancer mediates position-specific activation of Ultrabithorax in a single segment-wide stripe in an internal mesodermal layer of the embryo – the visceral mesoderm that envelops the embryonic midgut. Its minimal ~240bp core element turned out to be responsive to positional signalling from adjacent cells secreting Wingless/Wnt, Dpp/TGFbeta and Vein/Neuregulin (whereby the first term denotes the fly founder protein, followed by its mammalian ortholog; here and below). We thus discovered how the HOX gene Ultrabithorax maintains itself in the visceral mesoderm via an indirect autoregulatory loop through autocrine Dpp and Vein signalling and paracrine Wingless signalling from neighbouring cell groups. This ensures coordinate maintenance of Ultrabithorax expression in all cells that make up the segmental stripe surrounding the midgut. Positional inputs from these signals synergise at, and are integrated by, the Ultrabithorax midgut enhancer.
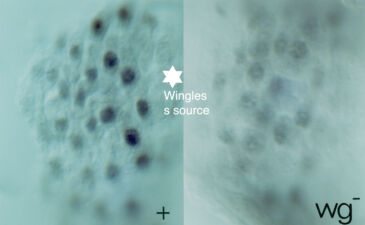
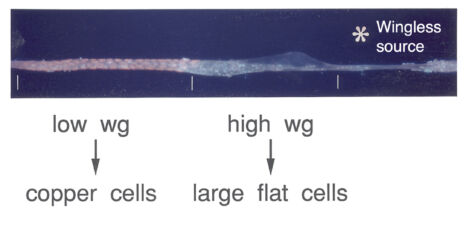
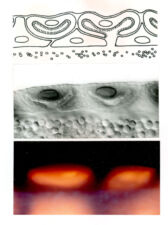
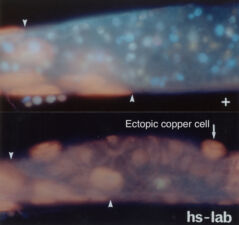
The same three signals cross over to the underlying endodermal cell layer, to specify a single stripe of Labial expression opposite the Ultrabithorax stripe though an intermediate endodermal target gene Dfos (Drosophilac-Fos). As in the case of Ultrabithorax, their inputs are integrated by a compact enhancer upstream of labial. Notably, Wingless signalling determines a gradient of Labial expression throughout the stripe, whereby the highest Labial levels are nearest to the Wingless signalling source. Subsequently, Labial specifies the differentiation of the so-called copper cells (a specialised cell type in the larval ‘stomach’) whereby the levels of Labial in these cells directly determines their size. These acid-secreting cells are required for larval growth, moulting and puparium formation. Through our work, the embryonic midgut became known as a paradigm for inductive signalling across two germ layers.
Relevant references:
- Bienz, M., Tremml, G. (1988)
Domain of Ultrabithorax expression in Drosophila visceral mesoderm from autoregulation and exclusion.
Nature 333(6173): 576-8 - Tremml, G., Bienz, M. (1989)
Homeotic gene expression in the visceral mesoderm of Drosophila embryos.
EMBO J 8(9): 2677-85 - Tremml, G., Bienz, M. (1989)
An essential role of even-skipped for homeotic gene expression in the Drosophila visceral mesoderm.
EMBO J 8(9): 2687-93 - Immerglück, K., Lawrence, P.A., Bienz, M. (1990)
Induction across germ layers in Drosophila mediated by a genetic cascade.
Cell 62(2): 261-8 - Thüringer, F., Bienz, M. (1993)
Indirect autoregulation of a homeotic Drosophila gene mediated by extracellular signaling.
Proc Natl Acad Sci U S A 90(9): 3899-903 - Hoppler, S., Bienz, M. (1994)
Specification of a single cell type by a Drosophila homeotic gene.
Cell 76(4): 689-702 - Bienz, M. (1994)
Homeotic genes and positional signalling in the Drosophila viscera.
Trends Genet 10(1): 22-6 - Hoppler, S., Bienz, M. (1995)
Two different thresholds of wingless signalling with distinct developmental consequences in the Drosophila midgut.
EMBO J 14(20): 5016-26 - Pöpperl, H., Bienz, M., Studer, M., Chan, S.K., Aparicio, S., Brenner, S., Mann, R.S., Krumlauf, R. (1995)
Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx.
Cell 81(7): 1031-42 - Yu, X., Hoppler, S., Eresh, S., Bienz, M. (1996)
decapentaplegic, a target gene of the wingless signalling pathway in the Drosophila midgut.
Development 122(3): 849-58 - Riese, J., Tremml, G., Bienz, M. (1997)
D-Fos, a target gene of Decapentaplegic signalling with a critical role during Drosophila endoderm induction.
Development 124(17): 3353-61 - Yu, X., Riese, J., Eresh, S., Bienz, M. (1998)
Transcriptional repression due to high levels of Wingless signalling.
EMBO J 17(23): 7021-32 - Szüts, D., Eresh, S., Bienz, M. (1998)
Functional intertwining of Dpp and EGFR signaling during Drosophila endoderm induction.
Genes Dev 12(13): 2022-35 - Szüts, D., Bienz, M. (2000)
An autoregulatory function of Dfos during Drosophila endoderm induction.
Mech Dev 98(1-2): 71-6
- Bienz, M. (1996)
Induction of the endoderm in Drosophila.
Seminars Cell Develop Biol 7113-119