 |
Clathrin-adaptor appendage domains
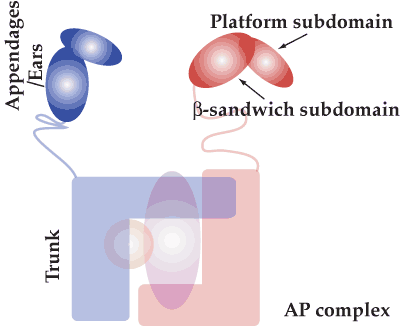
Appendage/ear domains recruit accessory proteins (see previous page) and are structurally and functionally conserved not merely amongst heterotetrameric AP complexes but also in subunits of the COPI coat complex and in GGAs (Golgi-localized, gamma-ear-containing, Arf-binding family of protein). There are 2 subdomains, a platform and a beta-sandwich (see illustration). In the movies below the overall structural conservation of binding sites is illustrated. The appendages of GGAs and the gamma subunit of AP-1 only have the beta-sandwich subdomain and they also bind peptide ligands. This has now led to a re-evaluation of possible peptide binding to beta-sandwich domains of other appendages and thus of accessory protein recruitment. This work is still ongoing. These pages are written to accompany a review by Mills et al 2004. |
 |
Please choose next page from the side menu
You are welcome to take from these pages as you wish and modify them, just acknowledge the source please.
|