 |
Recent Publications
Paul, D., Stern, O., Vallis, Y., Dhillon, J., Buchannan, A., McMahon, H. (2023) Cell surface protein aggregation triggers endocytosis to maintain plasma membrane proteostasis. Nature Communications (article)
Henri-Fran�ois Renard, Mijo Simunovic, Jo�l Lemi�re, Emmanuel Boucrot, Maria Daniela Garcia-Castillo, Senthil Arumugam, Val�rie Chambon, Christophe Lamaze, Christian Wunder, Anne K. Kenworthy, Anne A. Schmidt, Harvey T. McMahon, C�cile Sykes, Patricia Bassereau & Ludger Johannes (2015) Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature. 2015 Jan 22;517(7535):493-6. doi: 10.1038/nature14064. Epub 2014 Dec 17. PMID: 25517096
(abstract) (pdf including supplementary figures)
This Nature letter extends the observations of the article below, providing evidence that bacterial toxins, shiga and cholera, take advantage of an endophilin-dependent endocytic pathway to enter cells. The paper also finds that endophilin-A2 acts together with dynamin and actin.
Emmanuel Boucrot, Antonio P. A. Ferreira, Leonardo Almeida-Souza, Sylvain Debard, Yvonne Vallis, Gillian Howard, Laetitia Bertot, Nathalie Sauvonnet & Harvey T. McMahon (2015) Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015 Jan 22;517(7535):460-5. doi: 10.1038/nature14067. Epub 2014 Dec 17. PMID: 25517094 (abstract) (pdf including supplementary figures) (Video1) (Video2) (Video3) (Video4) (Video5)
 |
This Nature article shows that endophilin-dependent endocytosis is a novel endocytic pathway distinct from clathrin-mediated endocytosis. This pathway is triggered by binding of ligands to cargo receptors, and requires the proteins dynamin and actin. It occurs primarily from the leading edges of cells where lamellipodin and the lipid PtdIns(3,4)P2 ensures endophilin enrichment. This form of endocytosis is shown to mediate the uptake of several physiological and disease-relevant receptors including G-protein-coupled receptors and receptor tyrosine kinases.
See also BAR Superfamily Home Page
|
Safa Lucken-Ardjomande H�sler, Yvonne Vallis, Helen E. Jolin, Andrew N. McKenzie and Harvey T. McMahon (2014) GRAF1a is a brain-specific protein that promotes lipid droplet clustering and growth, and is enriched at lipid droplet junctions. J. Cell Sci. 2014 Nov 1;127(21):4602-19. doi: 10.1242/jcs.147694. Epub 2014 Sep 4. PMID: 25189622; PMCID: PMC4215711 (abstract) (pdf including supplementary figures) (Video1, large version) (Video2, large version) (Video3, large version) (Video4, large version)
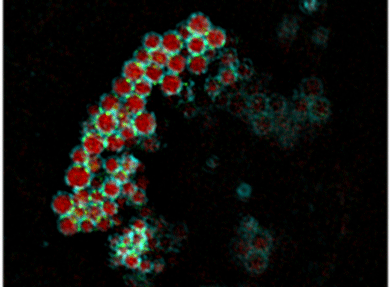 |
Lipid droplets are found in all cell types. Normally present at low levels in the brain, they accumulate in tumours and are associated with neurodegenerative diseases. However, little is known about the mechanisms controlling their homeostasis in the brain. We found that GRAF1a, the longest GRAF1 isoform (GRAF1 is also known as ARHGAP26), was enriched in the brains of neonates. Endogenous GRAF1a was found on lipid droplets in oleic-acid-fed primary glial cells. Exclusive localization required a GRAF1a-specific hydrophobic segment and two membrane-binding regions, a BAR and a PH domain. Overexpression of GRAF1a promoted lipid droplet clustering, inhibited droplet mobility and severely perturbed lipolysis following the chase of cells overloaded with fatty acids. Under these conditions, GRAF1a concentrated at the interface between lipid droplets (shown as green spots on the blue rings in picture on the left). Although GRAF1-knockout mice did not show any gross abnormal phenotype, the total lipid droplet volume that accumulated in GRAF1-/- primary glia upon incubation with fatty acids was reduced compared to GRAF1+/+ cells. These results provide additional insights into the mechanisms contributing to lipid droplet growth in non-adipocyte cells, and suggest that proteins with membrane sculpting BAR domains play a role in droplet homeostasis.
See also BAR Superfamily Home Page
|
Michael M. Kozlov, Felix Campelo, Nicole Liska, Leonid V. Chernomordik, Siewert J. Marrink and Harvey T. McMahon (2014) Mechanisms shaping cell membranes. Current Opinions in Cell Biology 29, 53-60. PMID: 24747171 (abstract) (pdf)
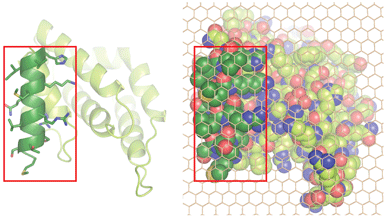 |
This is a cross between a paper and a review. It is essentially a reply to hypothesis by the Fletcher lab that membrane curvature is driven by protein crowding and not by insertions. It includes new data from Nicole Liska on curvature driven by crowding, new calculations on the effectiveness of this method by Kozlov and Campelo and new dynamic simulation of membranes by Marrink. Stachowiak et al 2012 claimed that protein crowding was the primary mechanism by which epsin deforms liposomes. Here we show that crowding can only make a contribution to bending but membrane insertion of the amphipathic helix is the primary factor.
The picture on the left shows the membrane interaction surface of epsin1 ENTH domain viewed from the membrane. The amphipathic helix, which inserts into the membrane is shown in dark green with amino acid side chains included. Space filled representation of the footprint with side chains included for the whole protein, overlaid with a carbon atom lattice (to facilitate area calculations), showing that the amphipathic helix occupies approximately 1/3rd of the total projection area.
See also BAR Superfamily Home Page
|
Tetsuya Takeda, Iain M. Robinson, Matthew M. Savoian, John R. Griffiths, Anthony D. Whetton, Harvey T. McMahon and David M. Glover (2013) Drosophila F-BAR protein Syndapin contributes to coupling the plasma membrane and contractile ring in cytokinesis. Open Biol. 2013 Aug 7;3(8):130081. doi: 10.1098/rsob.130081 PMID: 23926047, PMC3758542 (abstract) (pdf including supplementary figures)
 |
This paper reports for the first time that an F-BAR protein Syndapin (also called Pacsin) functions at the interface between membrane and the actomyosin contractile ring during cytokinesis of animal cells.
See also BAR Superfamily Home Page
|
Michael Meinecke, Emmanuel Boucrot, Gamze Camdere, Wai-Ching Hon, Rohit Mittal, and Harvey T McMahon (2013) Cooperative recruitment of Dynamin and BAR domain-containing proteins leads to GTP-dependent membrane scission. J. Biol. Chem. 288, 6651-6661 PMID:23297414, PMC3585104 (abstract) (pdf including supplementary figures)
 |
This paper reports the cooperativity between dynamin and N-BAR domain-containing proteins (amphiphysin and endophilin) for membrane binding and vesicle scission.
In cells we observed that
1. N-BAR protein overexpression leads to increased dynamin recruitment
2. RNAi against N-BAR-proteins leads to a reduction in dynamin recruitment.
3. We show that this RNAi of N-BAR proteins, directly leads to an inhibition of clathrin-coated vesicle (CCV) maturation.
4. The consequence of amphiphysin RNAi on CCV formation was more dramatic than that of endophilin RNAi. The clathrin-amphiphysin interaction inhibitor, pitstop, recapitulated the phenotype, showing that this drug ultimately is an inhibitor of CCV formation because it indirectly inhibits the recruitment of dynamin.
To understand the molecular basis of the observed cooperativity in vivo we developed an assay for membrane binding and scission using purified proteins and giant unilamellar vesicles (GUVs).
In vitro we observed that
1. Binding of either endophilin or amphiphysin to PIP2-containing membranes (GUVs) was enhanced when dynamin (or simply the dynamin- PRD) was bound to their SH3 domains.
2. In a similar manner dynamin interaction with membranes was stronger when its PRD binds the SH3 domain of either endophilin or amphiphysin.
3. This N-BAR dependence for dynamin recruitment to membranes for the first time recapitulates in vivo observations of adaptor dependence for dynamin recruitment to sites of endocytosis. We also recapitulate dynamin mediated vesicle scission in the presence of GTP and show this is enhanced in the presence of endophilin or amphiphysin. This new assay for vesicle scission is based on the reduction in the size of GUVs with time in the presence of GTP, indicating vesiculation.
We report a number of surprises:
1. RNAi against endophilin has no effect on transferrin endocytosis in BSC1 cells (Fig1E), which is contrary to implications in the literature, and implies that endophilin may have little to do with clathrin-coated vesicle maturation in cells.
2. Endophilin overexpression leads to increased dynamin membrane recruitment, while dynamin RNAi leads to reduced endophilin membrane recruitment. While these results reinforce the evidence for cooperativity between the molecules we also observed that endophilin RNAi appeared to lead to reduced dynamin expression (an unexpected result).
3. It has not been previously observed that effectors are needed to either recruit dynamin or to enhance dynamin activity in vitro. Our observations imply that GUV membranes more closely resemble the cellular environment in which these cooperative effects are observed.
See BAR Superfamily Home Page for details of endophilin and amphiphysin
See also Dynamin web pages.
|
Katia Cortese, Mark T. Howes, Richard Lundmark, Erica Tagliatti, Paola Bagnato, Annalisa Petrelli, Maria Bono, Harvey T. McMahon, Robert G. Parton and Carlo Tacchetti (2013) The HSP90 inhibitor geldanamycin perturbs endosomal structure and drives recycling ErbB2 and transferrin to modified MVBs/lysosomal compartments. Mol Biol Cell. 2013 Jan;24(2):129-44. doi: 10.1091/mbc.E12-04-0282. Epub 2012 Nov 14. PMID: 23154999, PMC3541960 (abstract) (pdf) (Movie1) (Movie2) (Movie3) (Movie4) (Movie5) A study on how the anti-cancer drug, geldanamycin, affects endocytic trafficking
Maria Jose Sanchez-Barrena, Yvonne Vallis, Menna R. Clatworthy, Gary J. Doherty, Dmitry B. Veprintsev, Philip R. Evans and Harvey T. McMahon (2012) Bin2 Is a Membrane Sculpting N-BAR Protein That Influences Leucocyte Podosomes, Motility and Phagocytosis. PLoS One. 2012;7(12):e52401. doi: 10.1371/journal.pone.0052401. Epub 2012 Dec 20. PMID: 23285027 PMCID: PMC3527510 (abstract) (pdf including supplementary figures) (Movie 1) (Movie2)
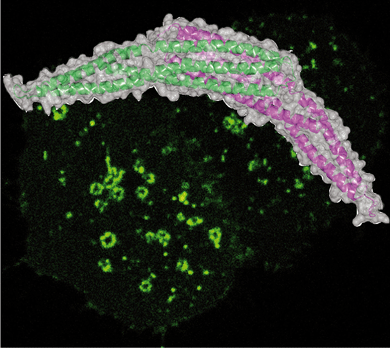 |
This paper reports the structure and functional analysis of Bin2/BRAP BAR domain-containing protein
Bin2 is only expressed in leucocytes and we have found it to be implicated in cell motility, adhesion and phagocytosis that are controlled by actin and membrane remodelling processes. We have solved the structure of Bin2 N-BAR domain and we have shown that this domain enables the protein to tubulate membranes in vivo and in vitro. Compared to previously characterized N-BAR-containing proteins, Bin2 lacks an SH3 domain to bind dynamin and in contrast, it contains a poly-proline region which we show interacts with endophilin, and PIX. We have found that Bin2 is a new component of the podosomal machinery. Bin2 N-BAR is responsible for its targeting to these actin rich structures and its C-terminus permits Bin2 to interact with SH3 domain containing proteins such as alpha-PIX and Endophilin A2. Manipulation of Bin2 protein levels affects podosome formation as it has been shown for PIX and its binding partner PAK. Furthermore, Bin2 is at the leading edge of migrating leukocytes and favours cell movement. Additionally, Bin2 is also found at the phagocytic cup of macrophages and inhibits phagocytosis. In summary, Bin2 is a N-BAR protein enriched in leucocytes and implicated in actin rearrangements associated with leucocyte morphogenesis.
See BAR Superfamily Home Page for details of endophilin and amphiphysin
|
Emmanuel Boucrot, Adi Pick, Gamze Camdere, Nicole Liska, Emma Evergren, Harvey T. McMahon, Michael M. Kozlov (2012) Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell Mar 30;149(1):124-36. PMID: 22464325 (abstract) (pdf including supplementary materials and figures)
Violeta Chitu, Viorel Nacu, Julia F. Charles, William M. Henne, Harvey T. McMahon, Sayan Nandi, Halley Ketchum, Renee Harris, Mary C. Nakamura and E. Richard Stanley (2012) PSTPIP2 deficiency in mice causes osteopenia and increased differentiation of multipotent myeloid precursors into osteoclasts. Blood 120, 3126-3135. [Epub ahead of print Aug24] PMID: 22923495 (abstract) (pdf including supplementary figures)
Chun-Liang Lai, Christine C. Jao, Edward Lyman, Jennifer L. Gallop, Brian J. Peter, Harvey T. McMahon, Ralf Langen and Gregory A. Voth (2012) Membrane Binding and Self-Association of the Epsin N-Terminal Homology Domain. Journal of Molecular Biology 423, 800-817. PMID: 22922484 (abstract) (pdf)
Bjorn Moren, Claudio Shah, Mark T. Howes, Nicole L. Schieber, Harvey T. McMahon, Robert G. Parton, Oliver Daumke and Richard Lundmark (2012) EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol. Biol. Cell Apr;23(7):1316-29. PMID: 22323287, PMCID: PMC3315815 (abstract) (pdf including supplementary materials and figures) (smaller versions of movies S1 635KB, S2 258KB, S3A 5MB, S3B 3.6MB, S4A 6.2MB, S4B 3.8MB)(full size movies S1 2.4MB, S2 1.2MB, S3A 24.3MB, S3B 15.2MB, S4A 35.7MB, S4B 30.3MB)
Gary J. Doherty, Monika K. Ahlund, Mark T. Howes, Bjorn Moren, Robert G. Parton, Harvey T. McMahon and Richard Lundmark (2011) The endocytic protein GRAF1 is directed to cell-matrix adhesion sites and regulates cell spreading. Mol. Biol. Cell. Nov;22(22):4380-4389. PMID: 21965292 (abstract) (pdf including supplementary materials and figures) (supplementary Movies 1, 2, 3, 4, 5)
Artur Llobet, Jennifer L. Gallop, Jemima E. Burden, Gamze Camdere, Priya Chandra, Yvonne Vallis, Collin R. Hopkins, Leon Lagnado, Harvey T. McMahon (2011) Endophilin drives the fast mode of vesicle retrieval in a ribbon synapse. J. Neurosci. Jun 8;31(23):8512-9. PMID: 21653855(abstract) (pdf including supplementary materials and figures)
Jean-Philippe Richard, Evgenia Leikina, Ralf Langen, William Mike Henne, Margarita Popova, Tamas Balla, Harvey T McMahon, Michael Kozlov and Leonid Chernomordik (2011) Intracellular curvature generating proteins in cell-to-cell fusion. Biochemical J. Dec 1;440(2):185-193. Epub 2011 Sept 7. PMID: 21895608 PMCID: PMC3216009 (abstract) (pdf)
Sascha Martens, Harvey T. McMahon (2011) C2 domains and membrane fusion. Curr. Top. Membr. 68:141-59. PMID: 21771498 (pdf)
Harvey T. McMahon, Emmanuel Boucrot (2011) Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. Jul 22;12(8):517-33. doi: 10.1038/nrm3151. PMID: 21779028 (abstract) (pdf)
Emmanuel Boucrot, Harvey T. McMahon (2011) [Nucleation of clathrin-coated pits - 'membrane sculptors' at work]. Med. Sci. (Paris). Feb;27(2):122-5. Epub 2011 Mar 8. French. PMID: 21382316 (abstract) (pdf)
Misha M. Kozlov, Harvey T. McMahon and Leonid V. Chernomordik (2010) Protein-driven membrane stresses in fusion and fission. Trends Biochem. Sci. 35, 699-706. [Epub ahead of print Jul 15 2010] PubMed PMID: 20638285. (abstract) (pdf)
William Mike Henne*, Emmanuel Boucrot*^, Michael Meinecke, Emma Evergren,Yvonne Vallis, Rohit Mittal and Harvey T. McMahon^ (2010) FCHo Proteins are Nucleators of Clathrin-Mediated Endocytosis. Science 328, 1281-1284. (*joint first authors,^joint corresponding authors) (Published online 6 May 2010; 10.1126/science.1188462) (abstract) (pdf including supplementary materials and figures) (movie1, increased CCV budding with FCHo2 overexpression) (movie2, simulation of membrane bending by FCHo proteins) PMID: 20448150; PMCID: PMC2883440 (Faculty of 1000 Exceptional rating) (News and Views in Nature) (Science Signalling, editors' choice)(Nature Reviews in Molecular Cell Biology, Research Highlight)
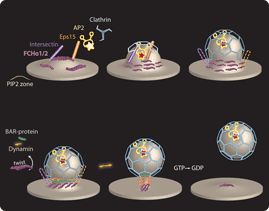 |
This is first membrane curvature effector protein to define sites where clathrin-coated vesicles will bud. It also appears to be the first 'coat-accessory protein' recruited recruited to the plasma membrane for the initiation of CCV formation here. Its expression levels correlate with the number of budding events and thus ligand uptake. The proteins are ubiquitously expressed and are shown to function in fibroblasts and in synapses.
See F-BAR homepage for structure details.
See endocytosis.org homepage for research on other endocytic proteins.
|
Misha M. Kozlov, Harvey T. McMahon and Leonid V. Chernomordik (2010) Protein-driven membrane stresses in fusion and fission. Trends Biochem Sci. 35, 699-706. [Epub ahead of print Jul 15 2010] PubMed PMID: 20638285. (abstract) (pdf)
Mark T. Howes, Matthew Kirkham, James Riches, Katia Cortese, Piers J. Walser, Fiona Simpson, Michelle M. Hill, Alun Jones, Richard Lundmark, Margaret R. Lindsay, Delia J. Hernandez-Deviez, Gordana Hadzic, Adam McCluskey, Rumasia Bashir, Libin Liu, Paul Pilch, Harvey McMahon, Phillip J. Robinson, John F. Hancock, Satyajit Mayor and Robert G. Parton (2010) Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. Aug 23;190(4):675-91. Epub 2010 Aug 16. PMID: 20713605; PMCID: PMC2928008. (abstract) (pdf including supplemental figures)
Ji-Young Youn, Helena Friesen, Takuma Kishimoto, William Mike Henne, Christoph F. Kurat, Wei Ye, Derek F. Ceccarelli, Frank Sicheri, Sepp D. Kohlwein, Harvey T. McMahon, Brenda J. Andrews (2010) Dissecting BAR domain function in the yeast Amphiphysins Rvs161 and Rvs167 during endocytosis. Mol. Biol. Cell. Sep;21(17):3054-3069. [Epub ahead of print Jul 7 2010] PMID: 20610658; PubMed Central PMCID: PMC2929998 (abstract) (pdf including supplemental material and figures)
Harvey T. McMahon, Misha Kozlov and Sascha Martens (2010) Membrane Curvature in Synaptic Vesicle Fusion and Beyond. Cell 140, 601-605. DOI 10.1016/j.cell.2010.02.017 PMID 20211126 (abstract) (pdf)
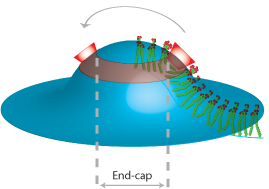 |
This essay presents the case why:
A. high transient membrane curvature is intrinsically fusogenic
B. synaptotagmin should be considered as a core fusion protein along with the SNARE proteins
C. all membrane fusion events likely involve a high curvature intermediate
D. many of the components in SNARE mediated fusion will contribute to curvature, including the SNARE proteins
E. a point source of fusion is created by high curvature and should lead to non-leaky membrane fusion.
See Synaptotagmin homepage for how synaptotagmin works
|
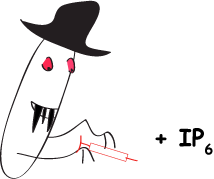
Rohit Mittal, Sew-Yeu Peak-Chew, Robert S Sade, Yvonne Vallis and Harvey T McMahon (2010) The acetyltransferase activity of the bacterial toxin YopJ of Yersinia is activated by eukaryotic host cell inositol hexakisphosphate. J. Biol. Chem. 285, 19927-199234. [Epub ahead of print 29Apr 2010] DOI 10.1074/jbc.M110.126581 PMID: 20430892 (abstract) (pdf including supplemental material and figures)
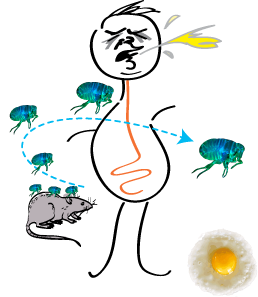 |
The bacterial toxin YopJ from Yersinia acetylates serine and threonine residues on important molecules in the MAP kinase and NFkappaB signalling pathways (see Mittal et al 2006, below). In this paper we now establish that YopJ from Yersinia and AvrA from Salmonella are activated by inositol hexakisphosphate (IP6) present in the eukaryotic target cell. Our results suggest that YopJ-family enzymes are quiescent in the bacterium where they are synthesized because bacteria lack IP6; once injected into mammalian cells by the pathogen these toxins bind host cell IP6, are activated and deregulate the MAPK and NFkappaB signaling pathways thereby subverting innate immunity.
See Black Plague Home Page that gives the background and further information on this paper. |
 |
Christine C. Jao, Balachandra G. Hegde, Jennifer L. Gallop, Prabhavati B. Hegde, Harvey T. McMahon, Ian S. Haworth and Ralf Langen (2010) Roles of amphipathic helices and the BAR domain of endophilin in membrane curvature generation. J. Biol. Chem. 285, 20164-70. [Epub ahead of print Apr 23, 2010] PMID: 20418375 (pdf)
(Alexander J. Groffen^, Sascha Martens^), Roc�o D�ez Arazola, L. Niels Cornelisse, Natalia Lozovaya, Arthur P. H. de Jong, Natalia A. Goriounova, Ron L. P. Habets, Yoshimi Takai, J. Gerard Borst, Nils Brose, (Harvey T. McMahon*, and Matthijs Verhage*) (2010) Doc2b Is a High-Affinity Ca2+ Sensor for Spontaneous Neurotransmitter Release. Science (article), 327, 1614-1618[First published online 11 February 2010 in Science Express Research Articles] [DOI: 10.1126/science.1183765] ^joint first and corresponding authors and *joint senior authors. PMID: 20150444 (pdf including supplementary material and figures) (CNCR news) (Faculty of 1000 Exceptional rating)
Here we have found a calcium sensor for spontaneous neurotransmitter release in the brain. It has a higher calcium affinity than synaptotagmin1 and thus can respond to much lower calcium rises. In vitro it promotes SNARE-dependent membrane fusion by promoting extreme local membrane curvature. This sensor competes with synaptotagmin, allowing us to predict that in vivo the properties of an individual vesicle fusion event will be a product of the different calcium-sensors present at the vesicle release site.
Felix Campelo, Gur Fabrikant, Harvey T. McMahon and Michael M. Kozlov (2009)
Modeling membrane shaping by proteins: Focus on EHD2 and N-BAR domains FEBS Lett. 584, 1830-1839. [Epub ahead of print 16Oct 2009] PMID: 19836393 (abstract) (pdf)
Ahead of the curve A Nature News and Views feature that highlights our work (16 July issue Nature 2009) (pdf)
Gary J. Doherty and Harvey T. McMahon (2009)
Mechanisms of Endocytosis. Annual Review in Biochemistry. 78, 857-902. [First published online as a Review in Advance on March 24, 2009]
PMID: 19317650 (abstract) (pdf)
Rohit Mittal and Harvey T. McMahon (2009) Arrestins as adaptors for ubiquitination in endocytosis and sorting. EMBO Rep. 10, 41-43. [Epub ahead of print Dec 2008] PMID: 19057574 (abstract) (pdf)
Richard Lundmark, Gary J. Doherty, Mark T. Howes, Katia Cortese, Yvonne Vallis, Robert G. Parton and Harvey T. McMahon (2008) The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Current Biology 18, 1802-1808. PMID: 19036340; PMC2726289; doi: 10.1016/j.cub.2008.10.044. (abstract) (pdf including supplementary figures)
A clathrin-independent tubulovesicular endocytic pathway dependent on the BAR-domain protein GRAF1.
Eberth A, Lundmark R, Gremer L, Dvorsky R, Koessmeier KT, McMahon HT, and Ahmadian MR. (2009) A BAR domain-mediated autoinhibitory mechanism for RhoGAPs of the GRAF family. Biochem. J. 417, 371-377. PMID: 18954304 (abstract) (pdf including supplementary figures)
Kenneth L. Madsen, Jacob Eriksen, Laura Milan-Lobo, Daniel S. Han, Masha Y. Niv, Ina Ammendrup-Johnsen, Ulla Henriksen, Vikram K. Bhatia, Dimitrios Stamou, Harald H. Sitte, Harvey T. McMahon, Harel Weinstein and Ulrik Gether (2008) Membrane Localization is Critical for Activation of the PICK1 BAR Domain. Traffic 9, 1327�1343. PMID: 18466293
(abstract) (pdf including supplementary material)
Kara L. Lynch, Roy R.L. Gerona, Dana M. Kielar, Sascha Martens, Harvey T. McMahon and Thomas F.J. Martin (2008) Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion. Molecular Biology of the Cell 19, 5093-5103. [Epub ahead of print Sept17 2008] PMID: 18799625 (abstract) (pdf including supplementary material) (Faculty of 1000 Biology Reviewed)
Richard Lundmark, Gary J. Doherty, Yvonne Vallis, Brian J. Peter and Harvey T. McMahon (2008) Arf family GTP loading is activated by, and generates, positive membrane curvature. Biochemical J. 414, 189-194. doi:10.1042/BJ20081237 PMID: 18597672 (abstract) (pdf) (commentary by Julia Donaldson, NIH)
Felix Campelo, Harvey T. McMahon and Misha M. Kozlov (2008) The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys J. 95, 2325-2339. [Epub ahead of print May 30] doi:10.1529/biophysj.108.133173, PMID: 18515373 (abstract) (pdf)
Sascha Martens and Harvey T. McMahon (2008) Mechanisms of membrane fusion: disparate players and common principles. Nature Reviews Molecular Cell Biology, 9, 543-556 (July issue). Advance online publication, 21 May 2008 | doi:10.1038/nrm2417, PMID: 18496517 (abstract) (pdf)
Gary J. Doherty and Harvey T. McMahon (2008)
Mediation, Modulation, and Consequences of Membrane-Cytoskeleton Interactions.
Annual Review in Biophysics 37, 65-95. PMID: 18573073 First published online as a Review in Advance on December 3, 2007
(doi:10.1146/annurev.biophys.37.032807.125912)
(pdf) (Ann Rev reprint)
Lene E. Olesen, Eva M. Schmid, Marijn G. J. Ford, Yvonne Vallis, M. Madan Babu, Peter Li, Ian G. Mills, Philip R. Evans, (Harvey T. McMahon and Gerrit J.K. Praefcke) (2008) Solitary and repetitive binding motifs for the AP2 complex alpha-appendage in amphiphysin and other accessory proteins. J. Biol. Chem. 283, 5099-5109. PMID: 17986441 First published on line in 2007, JBC Papers in Press, November 6, 2007, DOI 10.1074/jbc.M708621200 (abstract)
(pdf and supplementary material)
Oliver Daumke, Richard Lundmark, Yvonne Vallis, Sascha Martens, P. Jonathan G. Butler and Harvey T. McMahon (2007) Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature, 449 923-927. PMID: 17914359 (doi:10.1038/nature06173, online 3 Oct issue). (pdf) (abstract) (Supplementary Figures) (coordinates of proposed EHD oligomer, made from 4 dimers) (Faculty of 1000 - evaluation 6.5)
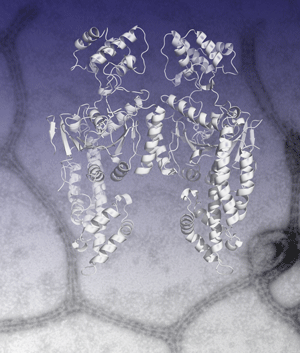 |
Dynamin superfamily NTPase members actively remodel membranes but molecular details are sparse. Here we present the structural details of EHD2 NTPase. The protein is a dimer in solution (and in the crystal) and oligomerises around membranes. We identify a highly curved membrane binding region and show when an EHD oligomer is formed how there will be perpendicular curvature stresses on a membrane surface which may result in remodelling of membranes by EHD oligomeric rings. This is the first crystal structure of a 'close' mammalian dynamin homologue and it is also the first crystal structure of an EH domain. In the EHD dimer the major interaction site of each EH domains is occupied by internal PF motifs from the main body of the protein.
See EHD Home Page that is written to accompany this paper showing additional information and movies. |
Eva M. Schmid and Harvey T. McMahon (2007) Integrating molecular and network biology to decode endocytosis. Nature, 448, 883-888. (doi:10.1038/nature06031, 23Aug issue). PMID: 17713526 (pdf) (abstract) (Supplementary Material)
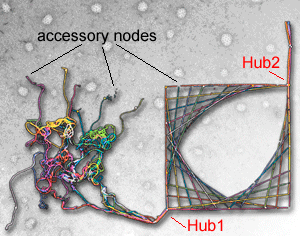 |
The strength of network biology is its ability to derive information about biology without a priori mechanistic or molecular knowledge. We show here how a careful understanding of biology can refine the strengths of the network approach. We do this by taking a well-studied biological pathway, clathrin-mediated endocytosis, and show how the protein machinery is organised around hubs resulting in a pathway interactome where the hub proteins are recognised by their connectivity and their higher conservation over accessory proteins. This approach causes us to observe the design principles behind the pathway, with inherent flexibility and fidelity that allow clathrin-coated vesicles to contain many different types of cargo and to originate from many different membrane-bound compartments. Studying the connectivity of the network can also lead to an approximation of the time course of events. We propose that an appreciation of the overall organisation helps in the understanding of spatiotemporal dynamics in cellular systems and in experimental design and interpretation. We further propose that the principles we derive can be applied to other biological systems.
See Interactome Home Page that is written to accompany this paper. |
William Mike Henne, Helen M. Kent, Marijn G.J. Ford, Balachandra G. Hegde, Oliver Daumke, P. Jonathan G. Butler, Rohit Mittal, Ralf Langen, Philip R. Evans, and Harvey T. McMahon (2007) Structure and Analysis of FCHo2 F-BAR Domain: a Dimerising and Membrane Recruitment Module that Effects Membrane Curvature. Structure 15, 839-852. PMID: 17540576 doi:10.1016/j.str.2007.05.002 (pdf) (Supplementary material) (MRC highlight)
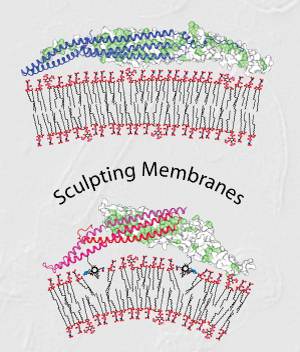 |
A spectrum of membrane curvatures exists within cells, and proteins have evolved different modules to detect, create and maintain these curvatures. Here we present the crystal structure of one such module found within human FCHo2. This F-BAR (extended FCH) module consists of two F-BAR domains, forming an intrinsically-curved all-helical anti-parallel dimer with a Kd of 2.5mM. The module binds liposomes via a concave face, deforming them into tubules with variable diameters of up to 130nm. Pulse EPR studies showed the membrane-bound dimer is the same as the crystal dimer, although the N-terminal helix changed conformation on membrane binding. Mutation of a phenylalanine on this helix partially attenuated narrow tubule formation, and resulted in a gain of curvature sensitivity. This structure shows a distant relationship to curvature-sensing BAR modules, and suggests how similar coiled-coil architectures in the BAR superfamily have evolved to expand the repertoire of membrane sculpting possibilities.
See BAR Superfamily Home Page These pages have been written to accompany our work on membrane curvature |
Sascha Martens, Michael M. Kozlov and Harvey T. McMahon (2007) How Synaptotagmin Promotes Membrane Fusion. Science. Published online 3 May 2007; 10.1126/science.1142614
Print Version: Science 2007, 316, 1205-1208 (25May) PMID: 17478680 (pdf including Supplemental material) (abstract from Science Website) (reprint from Science Website) (Highlight in Nature Structure and Molecular Biology: Syts throw a curve, 14, 467 (2007) doi:10.1038/nsmb0607-467 (pdf)) (Highlight in October 15 issue 2007 Journal of Physiology)
This paper shows that synaptotagmin-1, the major calcium sensor for synaptic vesicle exocytosis, induces extreme positive membrane curvature upon calcium-induced binding to membranes. It does this by inserting hydrophobic residues into the hydrophobic phase of the membrane. It has previously been shown that synaptotagmin-1 has to bind to membranes in order to promote synaptic vesicle exocytosis but the downstream effect of this membrane binding was unknown. We were thus the first to assign a functional outcome to calcium-induced membrane-binding by synaptotagmin and show that it is the extreme curvature induced by this protein that leads to membrane fusion. We went on to show that other synaptotagmin-related molecules also induce membrane curvature and showed that the calcium-dependent induction of membrane curvature by synaptotagmin-1 is essential for the promotion of SNARE-dependent membrane fusion. According to our model the extreme membrane curvature induced by synaptotagmin-1 results in the induction of curvature stress thereby reducing the high energy barrier for membrane fusion. Synaptotagmin-related molecules with multiple linked C2 domains are ubiquitous and our results suggest that the promotion of membrane fusion by multiple C2 domain containing proteins by the induction extreme positive curvature is a widespread phenomenon in biology. See Synaptotagmin Home Page
Anne Burtey, Eva M. Schmid, Marijn G.J. Ford, Joshua Z. Rappoport, Mark G.H. Scott, Stefano Marullo, Sanford M. Simon, Harvey T. McMahon and Alexandre Benmerah (2007) The conserved IVF motif couples activation state and endocytic functions of beta-arrestins. Traffic 8, 914-931(Epub 2007 Jun 4) PMID: 17540576 (pdf) (abstract)
Beta-arrestins play a central role in the regulation of G protein coupled receptors (GPCRs) and in their targeting for endocytosis. We previously solved the structure of the C-terminus of beta2-arrestin with beta-adaptin (Schmid et al 2006, see below). The adaptin interaction drives the recruitment of beta-arrestins-GPCR complexes into clathrin-coated pits. This paper describes in vitro and in vivo studies on arrestin dynamics.
Mittal, R., Peak-Chew, S-Y. and McMahon, H.T. (2006) Acetylation of MEK2 and IkB kinase (IKK) activation loop residues by YopJ inhibits signalling. PNAS 103, 18574-18579. PMID: 17116858 (abstract) (pdf) (Sup Figs 4-7) (Faculty of 1000 -exceptional factor 9).
This paper shows that the Yersinia toxin YopJ blocks the MAP kinase pathway and the NF-kB pathway by acetylation of serine residues in MEKs and IKKs respectively. Previously acetylation has been shown for lysine residues but here we show acetylation directly by the Yersinia toxin on serine and threonine residues in the activation loops of these kinases. These results raise the possibility that this type of acetylation may occur under non-pathogenic conditions and may regulate protein function in eukaryotic cells.
Schmid, E.M., Ford, M.G.J., Burtey, A., Praefcke, G.J.K., Peak-Chew, S-Y., Mills, I.G., Benmerah, A. and McMahon, H.T. (2006) Role of the AP2 beta-appendage hub in recruiting partners for clathrin-coated vesicle assembly. PLoS Biology 4(9), e262. PMID: 16903783 DOI: 10.1371/journal.pbio.0040262 (pdf) (sup.pdf)
This paper demonstrates the power of a multidisciplinary approach to understand the process of clathrin-mediated endocytosis. The investigation at first focuses on a comparison of the AP2 appendage interactors. By using a mass spectrometry-based proteomic approach we conclude that the “second ear” (the beta-appendage) helps widen the interaction repertoire of the AP2 complex and increases the avidity of some interaction partners. X-ray crystallography allowed us to identify two binding sites on the b-appendage, one on the top and the other on the side of the appendage, and from the structures we can see the essential interacting residues. Thus we can identify potential appendage binding motifs in other ligands. We also found a new mode of appendage interaction, where the top site interacting peptide from beta-arrestin undergoes a conformational change into a helix upon binding to the beta-appendage. This is again used by other ligands and the combination of sequence motif and structural modification gives an extra level of specificity to the interaction.
 |
Painting by an art student, Alex McCuish from Hills Road Sixth Form College, Cambridge UK, of the protein interaction network underlying nerve function.
See Interactome Home Page |
This paper gives us insights into the overall design principles behind clathrin-mediated endocytosis.
Firstly, we can give a reasoning why two appendages instead of one are needed: proteins that bind to both appendages have a higher chance of being recruited. Once recruited they have reduced off-rates and therefore are more likely to stay at the site of action where they are needed and will only be replaced by higher affinity binders. Such higher affinity binders can be proteins which have more than one interaction motif for the appendages and can therefore crosslink AP2’s on the membrane via binding to multiple appendages from different AP2 complexes.
Secondly: in the progression of endocytosis there is a maturation of hubs and while ligands may initially be recruited to sites of endocytosis by interactions with AP2 appendages, yet, to be incorporated in clathrin-coated vesicles they must need to change partners and become attached to clathrin coat.
Thirdly: to maintain reversibility and dynamic instability in the process we should consider the build up of protein interactions as a network where a combination of the reaching a critical concentration (through clustering) and the energy input step defines the point of no return. We therefore see the AP2 complex and clathrin as the major control centres of clathrin-mediated endocytosis, the hubs in a dynamic pathway-network.
Finally: we propose a new type of protein interaction that cannot be described by affinities or avidities because it is a semi-solid state interaction, and to describe this we coin the term, ‘Matricity’. This describes the interaction of a clathrin coat with its membrane attachment proteins and cargo adaptors.
Gallop, J.L., Jao, C.C., Kent, H.M., Butler, P.J.G., Evans, P.R., Langen, R. and McMahon, H.T. (2006) Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 25, 2898-2910. PMID: 16763559 (abstract) (pdf) (Sup Figures) (Sup Methods)
The crystal structure of endophilin BAR domain and electron spin resonance analysis of the N-terminal amphipathic helix in the presence of membranes. This paper shows how the N-BAR domain (amphipathic helix plus BAR domain) works as a functional unit to promote dimerisation and membrane curvature generation. An insert in the middle of the BAR domain, defines an endophilin/nadrin subfamily of BAR domains, where part of this insert forms another short amphipathic helix and also contributes to specificity of binding. See Endophilin Home Page
McMahon, H.T. and Gallop J.L. (2005) Membrane curvature and mechanisms of dynamic cell remodelling. Nature 438, 590-596. (invited review) (pdf)
Exploratory review on the role of proteins in dynamically changing membrane conformation and the downstream effects of these changes. We address different mechanisms of curvature generation and how these interact to orchestrate membrane trafficking. See BAR Superfamily Home Page
Higgins, M and McMahon, H.T. (2005) In vitro reconstitution of discrete stages of dynamin-dependent endocytosis. Methods Enzymology 404, 597-611. (pdf)
Gallop, J.L., Butler, P.J.G. and McMahon H.T. (2005) Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature 438, 675-678. (pdf) (supplementary figures)
Both endophilins and CtBP/BARS have been proposed to have lysophosphatidic acid acyl transferase (LPAAT) activities that can make phosphatidic acid in membranes. For endophilins this activity is thought to change the bilayer asymmetry in such a way that negative membrane curvature at the neck of a budding vesicle will be stabilised. Here we show that the LPAAT activities are contaminants associating with these proteins during purification. This now prompts a re-evaluation of the functions of these proteins. See Endophilin Home Page
Jochusch, W.J., Praefcke, G.J.K., (McMahon, H.T. and Lagnado, L.) (2005) Clathrin-dependent and clathrin-independent retrieval of synaptic vesicles in retinal bipolar cells. Neuron 46, 869-878. (abstract) (pdf).
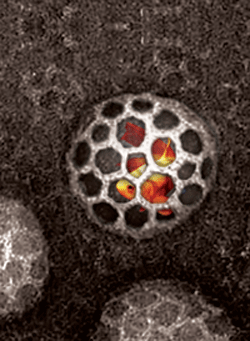 |
Endocytosis in central neurons can frequently be separated into two kinetic components. Here we show that the fast component in retinal bipolar cells (time constant of 1s) is clathrin-independent and dynamin dependent. The slow component (time constant of 10s) is AP2 adaptor, clathrin and dynamin-dependent and thus is the canonical clathrin-mediated endocytic pathway. |
Gallop, J.L. and McMahon H.T. (2005) BAR domains and membrane curvature: bringing your curves to the BAR. Biochem. Soc. Symp. 72, 223-231. (pdf)
Do BAR domains create membrane subdomains and how can BAR domains influence membrane trafficking? This review covers the significance of, and ways, in which BAR domains function in various contexts.
Carlton, J.G., Bujny, M.V., Peter, B.J., Oorschot, V.M.J., Rutherford, A., Arkell, R.S., Klumperman, J., McMahon, H.T. and Cullen, P.J. (2005) Sorting nexin-2 is associated with tubular elements of the early endosome, but is not essential for retromermediated endosome-to-TGN transport. J. Cell Sci. 118, 4527-4539. (pdf)
A continuation of the collaboration with Pete Cullen, Bristol, on the role of sorting-nexins in membrane trafficking. The BAR domain of sorting-nexin2 does not tubulate membranes but is sensitive to curvature and in vivo sorting-nexin2 localises to tubular elements of endosomes.
Praefcke, G.J.K., Ford, M.G.J., Schmid, E.M., Olesen, L.E., Gallop, J.L., Peak-Chew, S-Y., Vallis, Y., Madan Babu, M., Mills, I.G. and McMahon, H.T. (2004) Evolving nature of the AP2 alpha-appendage hub during clathrin-coated vesicle endocytosis. EMBO J. 23, 4371-4383. PMID: 15496985 (abstract) (pdf) (movie of AP2 alpha-appendage with synaptojanin peptides) (supplementary material) (see also cover of same issue) (Highlight in Science 2004, v306, p1103, pdf)
 |
This is the first of a series of network papers on trying to understand the making of a clathrin-coated vesicle as a 'series of many parallel processes'. In protein interaction networks, hubs are proteins that have disproportionately large numbers of interaction partners. For these hubs to function in a biological process it would seem that there must be sequential or spatial ordering of the interactions. In this paper, where we treat clathrin-mediated endocytosis as a module of a network, we uncover how the AP2 alpha-appendage works as an interaction hub after being concentrated at sites of endocytosis. These concentrated appendages provide a multivalent binding platform (hub) for interaction partners. Thus the partners are represented according to their relative affinities and concentrations. In this paper we also discuss the biological basis for the temporal nature of this hub and for the directionality imposed by the system. This has implications for all biological networks where similar principles are likely to underlie their functions.
(AP2 alpha-appendage network hub used on journal cover Large version of EMBO J. Cover and Cover text)
See also adaptor appendage domain web pages
 |
Agnes Saint-Pol, Belen Yelamos, Mohamed Amessou, Ian G. Mills, Marc Dugast, Daniele Tenza, Peter Schu, Claude Antony, Harvey T. McMahon, Christophe Lamaze and Ludger Johannes (2004) Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev. Cell 6, 525-538. (abstract) (pdf)
This paper builds on our 2003 epsinR paper by Mills et al, where we found that epsinR overexpression had a trafficking defect in the uptake of TGN46 antibodies. Here we define epsinR as a component of a vesicle budding step in retrograde transport from endosomes to the TGN. This pathway is used for the retrieval of cargo (including TGN38/46 and mannose-6-phosphate receptors) from endosomes and Shiga toxin can also traffic to the TGN via this pathway.
Carlton, J., Bujny, M., Peter, B.J., Oorschot, V.M., Rutherford, A., Mellor, H., Klumperman, J., McMahon, H.T. and Cullen, P.J. (2004) Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Current Biol. Oct 26;14(20):1791-800. (abstract) (pdf) (see also Research Roundup ‘Snx1 hugs the curves’ in JCB 2004 167, p581, pdf) (supplementary material and movies)
Arising from our observations that many sorting nexin proteins have BAR domains (see Peter et al 2004 below) we tested sorting nexin1 for membrane binding and curvature sensing and made appropriate mutations. We then collaborated with Peter Cullen (Bristol University) to test the in vivo significance for protein trafficking in cells. This paper reinforces our previous observation of the necessity for a lipid selective domain and a curvature sensing domain to work in tandem to localize proteins to high curvature on membranes (in this case endosomes) in the cell. This paper also demonstrates that overexpression of this sorting nexin leads to extreme tubulation of endosomes which disrupts all trafficking through this compartments, but an RNAi experiment shows that knock-down of the protein specifically disrupts the trafficking of mannose-6-phosphate receptors to the TGN and thus we propose that this receptor goes though via a tubule transporter which is formed/stabilised by sorting nexin 1.
See also BAR domain web pages
McMahon, H.T. and Mills, I.G. (2004) COP and clathrin-coated vesicle budding: different pathways, common approaches. Current Opinions in Cell Biol. 16, 379-391. (abstract) (pdf)
The remarkable similarities of appendage domains of COP and clathrin adaptors led us to look closely at the overall evolutionary relationships between COP and clathrin-coated vesicles. This review covers cargo recruitment, membrane curvature mechanisms and coat polymerization showing that common paradigms underpin these diverse pathways.
Web pages have been written to accompany this review including movies you can download
Tsuboi, T., McMahon, H.T. and Rutter, G.A. (2004) Mechanisms of dense core vesicle recapture following "kiss and run" ("cavicapture") exocytosis in insulin-secreting cells. J. Biol. Chem. 279 47115-47124. (abstract) (pdf) (Movies)
This paper is the result of a collaboration with Guy Rutter in Bristol University showing the dynamin-dependence of large-dense core vesicle endocytosis. This shows that the not only clathrin-mediated retrieval of vesicles but also the ?kiss and run? form of endocytosis is dynamin dependent. The stabilisation and closure of the fusion pore, through which transmitter is released, required the GTPase activity of dynamin.
See dynamin web pages
Praefcke, GJ.K. and McMahon, H.T. (2004) The Dynamin Superfamily: Universal membrane tubulation and fission molecules? Nature Reviews in Molecular Cell Biology 5, 133-147. (abstract) (pdf)
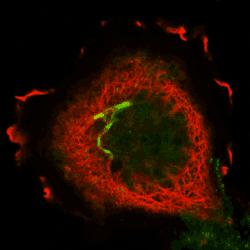 |
Here for the first time we define and classify the dynamin ‘Superfamily’ of proteins. All members have GTPase, oligomerisation and membrane targeting domains. The superfamily is subdivided into classical dynamins, dynamin-like proteins, Optic atrophy proteins (OPA1), Mx proteins and GBPs/atlastins. In this review we propose a common mechanism leading to membrane tubulation and/or fission that encompasses all superfamily members.
The COS cell on the left is overexpressing a GTPase mutant of dynamin1 (red, T65A-dynaminI) which forms a network of tubules at the end of which we can sometimes see a concentration of transferrin receptors in a fused compartment still accessible to the surface (green,transferrin).
The Dynamin web pages were written to accompany this review
|
Peter, B.J., Kent, H.M., Mills, I.G., Vallis, Y., Butler, P.J.G., Evans, P.R. and McMahon, H.T. (2004) BAR Domains as Sensors of Membrane Curvature: the Amphiphysin BAR Structure. published as a Research article on Science Express 26Nov 20
03; print version in Science, vol 303 Jan 23, 495-499. PMID: 14645856 (pdf: free from Science Web site) Supporting online
material Quicktime movie of the BAR structure (1.1 Mb) (pdf) See perspective (BAR domains Go on a Bender; Lee and Schekman) and cover in the same issue. See also Dispatch in Current Biology (Membrane Curvature: How BAR Domains Bend Bilayers) by Joshua Zimmerberg and Stuart McLaughlin vol 14, R250-R252 March23,2004. Faculty of 1000 (exceptional factor 9).
 |
In this paper we present the crystal structure of the BAR (Bin/Amphiphysin/Rvs) domain from Drosophila amphiphysin. The protein is a crescent-shaped dimer which binds to highly curved negatively charged membranes. Unexpectedly, we found structural similarity with arfaptin2, a G-protein effector which also binds and curves membranes. This similarity allowed us to search the sequence database where we found BAR domains in many proteins, including nadrins, sorting nexins, oligophrenins, centaurins, ICA69, and PICK1. In some of these proteins (N-BARs) the BAR is adjacent to an N-terminal amphipathic helix, and these N-BAR proteins can drive membrane curvature in vitro and in vivo. In other proteins the BAR is adjacent to a PH, PX, or other lipid binding domain, and in this context the BAR can sense membrane curvature, showing a strong preference for highly curved membranes (diameter <80nm). This curvature sensing effect is also seen with BAR domains alone, and is likely due to the curved shape of the broad membrane binding face. We propose that BAR domains act together with lipid binding domains to function as coincidence detectors for the correct degree of curvature and the correct lipid composition, and this will precisely localise proteins and any associated enzymatic activities to membrane sub-domains. The universal and minimal BAR domain is a dimerisation, membrane binding, and curvature sensing module found in many protein families.
See Bar domain web pages
 |
Stahelin, R. V., Long, F., Peter, B. J., Murray, D., De Camilli, P., McMahon, H. T. and Cho, W. (2003) Contrasting membrane interaction mechanisms of AP180 ANTH and Epsin ENTH domains. J. Biol. Chem. 278(31), 28993-28999. (abstract) (pdf)
This paper goes one step further than the paper by Ford et al below on epsin and membrane curvature. In the former paper we proposed that helix 'O' folds around the inositol headgroup of PtdIns(4,5)P2 and that the amphipathic nature of the helix allowed it to lie flat on the membrane surface and thus maximise its lipid displacement area leading to membrane curvature. We tested this hypothesis which was based on the ENTH structure and on EM data with point mutation along the helix, but in this paper, using pressure measurements, it is now shown that the epsin ENTH domain does indeed penetrate the lipid bilayer with helix 'O' playing a major role. The affinity of epsin ENTH domain for shortchain PtdIns(4,5)P2 is 850nM, while
the affinity for PtdIns(4,5)P2 containing liposomes is 23nM. Point mutations along the hydrophobic surface of helix O reduce the affinity to the same as that of short chain lipids showing that the hydrophobic interaction contributes greatly to the affinity for liposomes. The affinity of AP180 ANTH domain for the PtdIns(4,5)P2 head group IP3 is 28microM and its affinity for PtdIns(4,5)P2 containing liposomes is 27microM showing that this domain has no other interaction with lipids, consistent with the lack of an effect of binding on membrane curvature and on surface pressure measurements.
See ENTH versus ANTH domain web pages
Mills, I.G., Praefcke, G.J.K., Vallis, Y., Peter, B.J., Olesen, L.E., Gallop, J.L., Butler, P.J.G., Evans, P.R. and McMahon, H.T. (2003) EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking. J. Cell Biol. 160, 213-222. PMID: 12538641 (abstract) (pdf) (supplemental data)
If epsin is important for membrane bending under the clathrin lattice then there must be an epsin homologue for AP1/clathrin budding events. Here we describe a ubiquitous epsin1 homologue, which we call epsinR (for epsin-related protein). Endogenous epsinR in COS cells shows a TGN localisation and also puncta in the cytoplasm. Overexpression of lipid binding mutants cause disruption of the TGN to lysosome trafficking pathway (cathepsinD now goes to the cell surface) and also disruption of plasma membrane to TGN trafficking.
In addition to the cell biology in this paper we characterised epsinR binding to gamma-adaptin in the AP1 complex and to clathrin. We were the first to identify and characterise a gamma-adaptin binding sequence present in epsinR, gamma-synergin and Eps15. We identify the strong consensus sequence as being DFxDF where, in many cases, only one of the D residues is present.
See EpsinR home page
Kent, H.M., McMahon, H.T., Evans, P.R., Benmerah, A. and Owen, D.J. (2002) Gamma-adaptin appendage domain: Structure and binding site for Eps15 and gamma-synergin. Structure 10, 1139-1148. (abstract) (pdf)
In this paper we present the crystal structure of the gamma-adaptin appendage domain which is part of the AP1 complex. The appendage is very similar to both the alpha and beta appendages of the AP2 complex that we previously published except for the absence of a platform domain where the DxF motifs bind. We further indentify an interaction site on the gamma appendage for gamma ligands by point mutagenesis.
See movies of appendage domain structures
Ford, M.G.J., Mills, I.G., Peter, B.J., Vallis, Y., Praefcke, G.J.K., Evans, P.R. and McMahon, H.T. (2002) Curvature of clathrin-coated pits driven by epsin. Nature 419, 361-366. (abstract) (pdf) (supplemental data)
(see also News and Views in Nature, highlights in Nature Cell Biology (pdf), and in Nature reviews in molecular cell biology, and a mini review in Cell (Cell October 18, 2002: 111 (2):143-146) (summary)
This paper shows that epsin is able to deform membranes by binding to PtdIns(4,5)P2 and inserting an amphipathic helix into the membrane. In conjunction with clathrin polymerisation we propose that this drives the curvature of the nascent coated pit. We present the crystal structure of epsin ENTH domain bound to PtdIns(4,5)P2 with mutagenesis of the PtdIns(4,5)P2 binding site and the hydrophobic surface of helix '0'. PtdIns(4,5)P2 binding mutants are not recruited to the plasma membrane and inhibit AP2 adaptor localisation at the plasma membrane. This results in the inhibition of clathrin-mediated endocytosis. We also present the effect of epsin in tubulating liposomes and of epsin + clathrin in forming clathrin-coated pits on lipid monolayers.
See Epsin home page
See also protein spotlight (pdf)
Higgins, M.K. and McMahon, H.T. (2002) Snap-shots of clathrin-mediated endocytosis. (TIBS 27, 257-263. abstract) (pdf)
A review of electron microscopy techniques as applied to endocytosis.
Graham, M.E., O'Callaghan, D.W., McMahon, H.T. and Burgoyne, R.D. (2002) Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc. Natl. Acad. Sci. U S A. 99(10), 7124-7129. (abstract) (pdf)
Razzaq, A., Robinson, I.M., McMahon, H.T., Skepper, J.N., Su, Y., Zelhof, A.C., Jackson, A.P., Gay, N.J. and O'Kane, C.J. (2001) Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not
for synaptic vesicle endocytosis in Drosophila.
Genes Dev. 15(22), 2967-2979. (abstract) (pdf)
Deletion of the amphiphysin gene in Drosophila led to a defect in excitation-contraction coupling in muscle. This was analysed and we found that the muscle T-tubule system is not correctly developed. We show that this form of amphiphysin can tubulate lipids and suggest that this function is essential in the formation of the tubule network. This paper also highlights that amphiphysin function is not restricted to the synapse and also that it is not restricted to its interaction with dynamin. Thus amphiphysin has a different role in muscle than in the synapse. In the fly there does not appear to be a neuronal form of amphiphysin.
See Amphiphysin home page
Ford, M.G.J., Pearse, B., Higgins, M., Vallis, Y., Owen, D., Gibson, A., Hopkins, C.R., Evans, P.R. and McMahon, H.T. (2001) Simultaneous binding of PI (4,5)P2 and clathrin by AP180 causes nucleation of clathrin lattices
on membranes. Science 291, 1051-1055. (abstract) (pdf) (Also see cover , perspective (pdf)
and highlight 'coordinating entry' in same issue)
 |
This paper presents the crystal structure of CALM ANTH domain bound to PtdIns(4,5)P2 and demonstrates the critical role of AP180/CALM in clathrin recruitment to lipid membranes. A new assay has been developed whereby
clathrin polymerisation by AP180 on lipid monolayers can be visualised by electron microscopy. This paper highlights the role of PtdIns(4,5)P2 in the membrane in the initial stages of clathrin assembly/vesicle formation.
See AP180 home page |
Marks, B., Stowell, M., Vallis, Y., Mills, I., Gibson, A., Hopkins, C.R. and McMahon, H.T. (2001) GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410, 231-235. (abstract) (pdf) (see picture story in Nature Structural Biology, April issue 8, 301)
This paper shows that dynamin is a mechanochemical molecule and not a regulator of endocytosis (as had been previously proposed). We have made the first GTPase-'dead' mutants of dynamin and show that these do not accelerate endocytosis as predicted by models of classical GTPases, but instead vesicle scission is arrested and the vesicle neck is extended over time, eventually filling the cells with tubulated/invaginated plasma membrane. The effects of GTPase mutants of dynamin are monitored by electron microscopy and demonstrate that the change in pitch of the dynamin helix on GTP hydrolysis is prevented by the GTPase-dead mutation. We also identified another important mutation in this paper that prevents the conformational change in dynamin while maintaining a wild type GTP hydrolysis rate. As predicted from a mechanochemical model for dynamin's action, this mutant gives a severe endocytic block.
See Dynamin home page
Reim, K., Mansour, M., Varoqueaux, F., McMahon, H.T., Südhof, T., Brose, N. and Rosenmund, C. (2001) Complexins regulate a late step in Ca-dependent neurotransmitter release. Cell 104, 71-81. (abstract) (pdf)
The complexins were cloned by Harvey in 1995 and shown to bind to the core SNARE complex (see paper below). Mouse knock-outs of these proteins were made to determine their role in exocytosis. This paper is the culmination of the mice work and confirms our previous conclusion that complexins are indeed involved in a late stage of exocytosis. However the surprising result is that the mice have an altered calcium sensitivity of exocytosis implying a role for complexins here.
Wigge, P. and McMahon, H.T. (2000) Synaptic vesicle recycling: multiple pathways and functions in the nervous system (p204-214) in Endocytosis issue (ed Mark Marsh) of Frontiers in Molecular Biology series published by Oxford University Press (eds B.D.Hames and D.M.Glover).
A book chapter on the possible implication of endocytosis in synaptic function and synaptic plasticity.
Owen, D.J., Vallis, Y., Pearse, B.M.F., McMahon, H.T. and Evans, P.R (joint corresponding author) (2000) The structure and function of the B2-adaptin appendage domain. EMBO J. 19, 4216-4227. (abstract) (pdf)
Following on from our structure determination of the alpha-adaptin appendage domain we here present the crystal structure of the beta2-adaptin appendage domain. We demonstrate that despite a similar fold the ligand binding site has a different ligand specificity. The major binding partner for beta2-adaptin is clathrin and this paper shows that beta2-adaptin has at least 2 binding sites (one on the hinge and one on the appendage). This combination leads to a very efficient polymerisation of clathrin into cages in vitro and so this is proposed to be part of the role of the beta2-appendage domain.
See Adaptor home page
Marsh, M. and McMahon, H.T. (1999) The Structural Era of Endocytosis. Science 285, 215-220. (abstract) (pdf)
This was the first of a large number of reviews that cover the implication of structural analysis of endocytic proteins for our understanding of the biology.
Owen, D.J., Vallis, Y., Noble, M.E.M., Hunter, J.B., Dafforn, T.R., Evans, P.R., and McMahon, H.T. (1999). A structural explanation for the binding of multiple ligands by the alpha-adaptin appendage domain. Cell 97, 805-815. (abstract) (pdf)
The first crystal structure of the binding domain for accessory proteins on adaptors. We identified the binding site and a short peptide motif that binds to this site. This allowed us to identify new ligands for this domain simply by database trawling. We proposed that the appendage domain of adaptors is the linchpin for clathrin-mediated endocytosis, recruiting most of the accessory proteins via the same binding site. We do not know how this process is temporally regulated but the strength of interactions of the many ligands varies dramatically.
Stowell, M.H.B., Marks, B., Wigge, P., and McMahon, H.T. (1999) Nucleotide-dependent conformational changes in dynamin: Evidence for a mechanochemical molecular spring. Nature Cell Biol. 1, 27-32. (abstract) (pdf)
This was the first demonstration of dynamin's GTPase activity being stimulated under physiological conditions. We found that lipid nanotubules containing PtdIns(4,5)P2 could stimulate the GTPase activity by over 1000-fold. By EM we were able to observe a 2-fold increase in the pitch of the dynamin helix on GTP hydrolysis. This led us to propose a model by which this extension of the helix would force a nascent vesicle away from the parent membrane. This is a cooperative process and the energy from GTP hydrolysis of dynamin molecules in the helix is more than sufficient to break the membrane. Our subsequent Nature paper (2001) tested this hypothesis with several key mutants. The model stands the test of time but we would still like a direct measurement of the force produced by GTP hydrolysis.
Vallis, Y., Wigge, P., Marks, B., Evans, P.R., and McMahon, H.T. (1999) Importance of the PH domain of dynamin in clathrin-mediated endocytosis. Current Biol. 9, 257-260. (abstract) (pdf) (see also review in next issue)
In this paper we modelled the head group of PtdIns(4,5)P2 into the PH domain of dynamin and made some mutations to prevent binding. The key mutant K535A in the context of full-length dynamin was then shown to not support endocytosis of transferrin and EGF into cells. The in vivo recruitment of dynamin by amphiphysin was also tested in this paper where we show that cells expressing dynamin K535A could be partially rescued by a further mutation of the amphiphysin binding site of dynamin.
See Dynamin home page
McMahon, H.T. (1999). Endocytosis: An assembly protein for clathrin cages. Current Biol. 9, R332-335. (abstract)
The first and only review to date on the protein AP180.
Owen, D.J., Wigge, P. Vallis, Y., Moore, J.D.A., Evans, P.R. and McMahon H.T. (1998) Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation. EMBO J. 17, 5273-5285. (abstract) (pdf)
In this paper we presented the crystal structure of the major dynamin-binding domain of amphiphysin-2. This study allowed us to make specific mutations in dynamin that tested the role of amphiphysin in the recruitment of dynamin to sites of endocytosis (see Current Biology paper 1999). We also found that when dynamin was bound to amphiphysin it was held in a non-polymerised state and thus could be recruited at high concentrations to the neck of a forming vesicle awaiting the signal for release and helix formation and thus activation of its GTPase activity.
See Amphiphysin home page
Marks, B. and McMahon, H.T. (1998) Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Current Biol. 8, 740-749. (abstract) (pdf)
This was our initial entry into endocytosis with a study to find out if endocytosis is differently regulated to exocytosis. We found that calcium is the trigger for endocytosis (just as had been previously known for exocytosis). We showed that a target for the calcium was the calcium-dependent phosphatase, calcineurin. This phosphatase has a lower affinity for calcium than the exocytic trigger and so will sense 'residual' calcium after exocytosis (calcium that diffuses away from calcium hot-spots around sites of exocytosis) and therefore fits with the fact that endocytosis can occur in response to exocytosis in the living synapse. We found that by manipulation we could trigger endocytosis without exocytosis by using a low calcium stimulus, and by using an inhibitor of the calcineurin (cyclosporin) we could trigger exocytosis without endocytosis. We furthermore confirmed that the major target of calcineurin was the protein apparatus of clathrin-mediated endocytosis. This was the study that started us out on a path of wanting to understand the molecular machinery of clathrin-mediated endocytosis so that we could determine its regulation and specificity.
Wigge, P. and McMahon, H.T. (1998) The Amphiphysin Family and their role in Endocytosis at the Synapse. TINS 21, 339-344. (abstract) (pdf)
Wigge, P. and McMahon, H.T. (1998) Amphiphysins, in Guidebook to the Cytoskeletal and Motor Proteins published by Oxford University Press (eds Thomas E. Kreis and Ron Vale).
Wigge, P., Vallis, Y. and McMahon, H.T. (1997) Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Current Biol. 7, 554-560. (abstract) (pdf)
McMahon, H.T., Wigge, P. and Smith, C. (1997) Clathrin interacts specifically with amphiphysin and is displaced by dynamin. FEBS Lett. 413, 319-322. (abstract) (pdf)
Wigge, P., Köhler, K., Vallis, Y., Doyle, C.A., Owen, D., Hunt, S.P. and McMahon, H.T. (1997) Amphiphysin heterodimers: Potential role in clathrin-mediated endocytosis. Mol. Biol. Cell 8, 2003-2015. (abstract) (pdf)
|