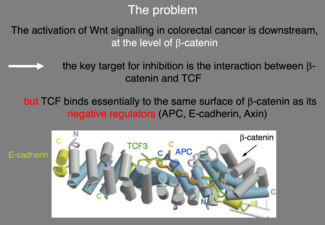
In the meantime, work by Polakis, Clevers, Vogelstein and others had revealed that dysregulated Wnt/beta-catenin signalling can be a major cancer driver, especially for colorectal cancer, and that hyperactive beta-catenin is a potent oncogene in many tissues. However, beta-catenin was thought to be undruggable, as there are no known enzymes to inhibit below beta-catenin. Indeed, the only known effectors downstream of beta-catenin were the TCF/LEF factors, which bind to an extensive surface of beta-catenin that is shared with beta-catenin antagonists such as APC and Axin.
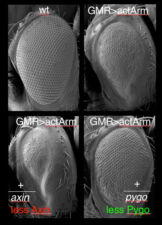
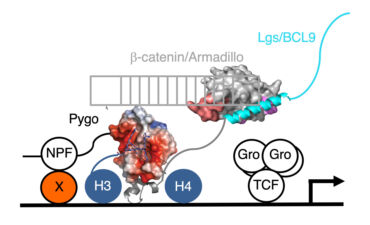
We therefore designed a suppressor screen based on activated Armadillo expressed in the fly eye, to identify new factors required for its activity, expecting some of the suppressor hits to be co-activators of TCF that are conserved in humans. We thus discovered Pygopus (Pygo1 and Pygo2 in humans), a nuclear factor essential for the activity of activated Armadillo throughout fly development. Molecularly, Pygo proteins use their PHD fingers to recognise histone H3 tail tri-methylated at lysine 4 (an epigenetic hallmark for active transcription) while also associating with Armadillo/beta-catenin through the Legless/BCL9 adaptor discovered by the Basler group in 2002.
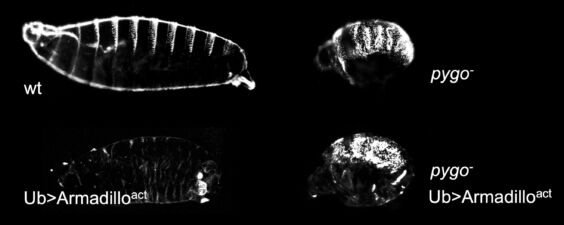
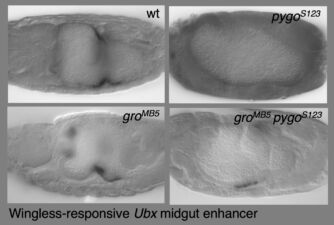
Unexpectedly, Drosophila Pygo turned out to be associated constitutively with Wingless-responsive loci through its chromatin-binding PHD finger. Remarkably, the requirement for pygo function can be overcome by high nuclear concentrations of Armadillo or by genetic deletion of the transcriptional co-repressor Groucho (known to bind TCF). We therefore proposed the ‘Armadillo-loading model’, according to which Pygo acts as an anti-repressor, by associating with Wingless-responsive genes in the absence of Wingless signalling to poise Wingless-responsive enhancers for signal activation. Pygo could thus capture Armadillo through the Legless/BCL9 adaptor and load it onto TCF by displacing the Groucho co-repressor (which, like its mammalian TLE orthologs, was known to keep Wnt-responsive genes inactive in the absence of Wingless/Wnt signalling).
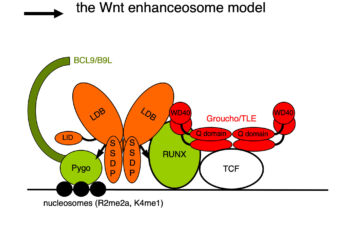
While our Armadillo-loading model incorporated everything we knew at the time about Pygo and other nuclear factors mediating Wnt-dependent transcription, it contained one glaring unknown – namely the physical link (‘factor X’) that guides Pygo to TCF target genes in the absence of Wnt signalling. Factor X was likely to bind to the N-terminal NPF signature motif of Pygo, essential for its function in flies and required for its binding to TCF target genes. However, identifying this NPF-binding factor proved to be a formidable challenge, which took several years to solve: unexpectedly, factor X turned out to be a complex between two proteins, which we called ChiLS. We discovered ChiLS in a proteomics screen as a complex that binds directly and specifically to the NPF motif of Pygo. ChiLS comprises a homo-dimer of a large protein called Chip/LIM-homeodomain binding protein (Chip/LDB) and a homo-tetramer of Single-strand DNA-binding protein (SSDP, also known as SSBP). The ChiLS complex was already known from mammalian haematopoietic cells in which it associates with the Locus Control Region of the beta-globin gene cluster and other remote enhancers, exerting key functions in haematopoiesis. ChiLS is tethered to its cognate enhancers by sequence-specific enhancer-binding proteins, either LIM homeodomain proteins to which it binds directly, or bHLH or GATA factors to which it binds via LIM-only (LMO) adaptors. In the murine intestine, Ldb1 was also known to function in the Wnt-dependent crypt stem cell compartment, whereas in fly imaginal discs, Chip and ssdp had been implicated in Notch and Wingless responses of remote transcriptional enhancers. Importantly, LIM-only 2 (LMO2) is an oncogene that, upon inappropriate expression in T cells, drives acute leukaemia by blocking their normal T cell differentiation programme.
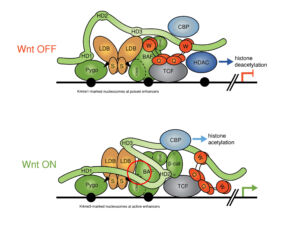
We discovered that ChiLS is the core component of a multi-protein complex termed the Wnt enhanceosome, which associates with the Ultrabithorax and labial midgut enhancers via Groucho-TCF to drive a Wnt-dependent transcriptional programme. ChiLS thus primes these HOX genes for responses to Wingless and its cooperating signals, and for ON-OFF switches that integrate their inputs with those from lineage-specific factors, including LIM and LMO proteins, GATA and bHLH factors. Its scaffold is BCL9/Legless, a large intrinsically disordered protein that links beta-catenin with Pygo through its direct binding to ChiLS and Groucho/TLE. Our work in flies and human cells thus revealed that the Wnt enhanceosome imparts context-specificity on Wnt-responsive selector genes, providing a molecular explanation for the context-dependence of TCF/LEF factors. Notably, Notch is one of the signals feeding into the Wnt enhanceosome, priming it for the OFF state and reinstalling Groucho/TLE-dependent repression on Wnt target genes upon cessation of Wnt signalling.
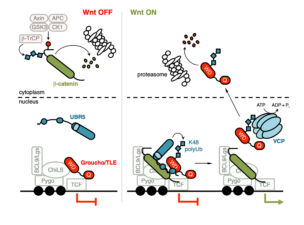
We also discovered Hyd/UBR5 as a HECT E3 ubiquitin ligase essential for Wnt-dependent derepression of Wnt target genes. In Drosophila imaginal discs, the hyd phenotype mimics that of pygo, and its function for Wingless responses is partially bypassed in the absence of groucho. In human cells, UBR5 ubiquitylates TLE during Wnt signalling, thereby relieving TLE-dependent repression of Wnt target genes, possibly via remodelling compacted chromatin.
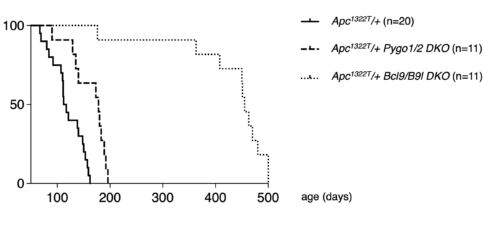
Our genetic analysis of the mouse intestine revealed that pygo and bcl9 synergise to drive stem cell-like transcriptional programmes in Apc mutant tumours while antagonising their cell differentiation. Remarkably, loss of Bcl9 essentially cured Apc mutant mice (developed by the Tomlinson group as the prime model for early-stage colorectal cancer) of their neoplastic disease, by suppressing tumourigenesis and restoring a near-healthy life span in these otherwise moribund mice. This indicated the potential of BCL9 as a therapeutic target at the early stages of colorectal cancer.
We have recently determined the architecture of the ChiLS core complex and the position of its Pygo-binding pocket and proposed a structural model, now validated by X-ray crystallography in collaboration with Wenqing Xu (ShanghaiTech University). This work will pave the way towards determining the structure of the complete Wnt enhanceosome.
Relevant references:
- Freeman, M., Bienz, M. (2001)
EGF receptor/Rolled MAP kinase signalling protects cells against activated Armadillo in the Drosophila eye.
EMBO Rep 2(2): 157-62 - Thompson, B., Townsley, F., Rosin-Arbesfeld, R., Musisi, H., Bienz, M. (2002)
A new nuclear component of the Wnt signalling pathway.
Nat Cell Biol 4(5): 367-73 - Townsley, F.M., Cliffe, A., Bienz, M. (2004)
Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function.
Nat Cell Biol 6(7): 626-33 - Thompson, B.J. (2004)
A complex of Armadillo, Legless, and Pygopus coactivates dTCF to activate wingless target genes.
Curr Biol 14(6): 458-66 - Townsley, F.M., Thompson, B., Bienz, M. (2004)
Pygopus residues required for its binding to Legless are critical for transcription and development.
J Biol Chem 279(7): 5177-83 - Hanson, K.K., Kelley, A.C., Bienz, M. (2005)
Loss of Drosophila borealin causes polyploidy, delayed apoptosis and abnormal tissue development.
Development 132(21): 4777-87 - Bray, S., Musisi, H., Bienz, M. (2005)
Bre1 is required for Notch signaling and histone modification.
Dev Cell 8(2): 279-86 - Bienz, M. (2006)
The PHD finger, a nuclear protein-interaction domain.
Trends Biochem Sci 31(1): 35-40 - de la Roche, M., Bienz, M. (2007)
Wingless-independent association of Pygopus with dTCF target genes.
Curr Biol 17(6): 556-61 - Mieszczanek, J., de la Roche, M., Bienz, M. (2008)
A role of Pygopus as an anti-repressor in facilitating Wnt-dependent transcription.
Proc Natl Acad Sci U S A 105(49): 19324-9 - de la Roche, M., Worm, J., Bienz, M. (2008)
The function of BCL9 in Wnt/beta-catenin signaling and colorectal cancer cells.
BMC Cancer 8: 199 - Fiedler, M., et al. (2008)
Decoding of methylated histone H3 tail by the Pygo-BCL9 Wnt signaling complex.
Mol Cell 30(4): 507-18 - Miller, T.C., Rutherford, T.J., Johnson, C.M., Fiedler, M., Bienz, M. (2010)
Allosteric remodelling of the histone H3 binding pocket in the Pygo2 PHD finger triggered by its binding to the B9L/BCL9 co-factor.
J Mol Biol 401(5): 969-84 - de la Roche, M., Rutherford, T.J., Gupta, D., Veprintsev, D.B., Saxty, B., Freund, S.M., Bienz, M. (2012)
An intrinsically labile α-helix abutting the BCL9-binding site of β-catenin is required for its inhibition by carnosic acid.
Nat Commun 3: 680 - Miller, T.C., Mieszczanek, J., Sánchez-Barrena, M.J., Rutherford, T.J., Fiedler, M., Bienz, M. (2013)
Evolutionary adaptation of the fly Pygo PHD finger toward recognizing histone H3 tail methylated at arginine 2.
Structure 21(12): 2208-20 - Miller, T.C., Rutherford, T.J., Birchall, K., Chugh, J., Fiedler, M., Bienz, M. (2014)
Competitive binding of a benzimidazole to the histone-binding pocket of the Pygo PHD finger.
ACS Chem Biol 9(12): 2864-74 - de la Roche, M., Ibrahim, A.E., Mieszczanek, J., Bienz, M. (2014)
LEF1 and B9L shield β-catenin from inactivation by Axin, desensitizing colorectal cancer cells to tankyrase inhibitors.
Cancer Res 74(5): 1495-505 - Fiedler, M., Graeb, M., Mieszczanek, J., Rutherford, T.J., Johnson, C.M., Bienz, M. (2015)
An ancient Pygo-dependent Wnt enhanceosome integrated by Chip/LDB-SSDP.
Elife 4 - Flack, J.E., Mieszczanek, J., Novcic, N., Bienz, M. (2017)
Wnt-Dependent Inactivation of the Groucho/TLE Co-repressor by the HECT E3 Ubiquitin Ligase Hyd/UBR5.
Mol Cell 67(2): 181-193.e5 - van Tienen, L.M., Mieszczanek, J., Fiedler, M., Rutherford, T.J., Bienz, M. (2017)
Constitutive scaffolding of multiple Wnt enhanceosome components by Legless/BCL9.
Elife 6 - Renko, M., Fiedler, M., Rutherford, T.J., Schaefer, J.V., Plückthun, A., Bienz, M. (2019)
Rotational symmetry of the structured Chip/LDB-SSDP core module of the Wnt enhanceosome.
Proc Natl Acad Sci U S A 116(42): 20977-20983 - Mieszczanek, J., van Tienen, L.M., Ibrahim, A.E.K., Winton, D.J., Bienz, M. (2019)
Bcl9 and Pygo synergise downstream of Apc to effect intestinal neoplasia in FAP mouse models.
Nat Commun 10(1): 724 - Wang, H., Bienz, M., Yan, X.X., Xu, W. (2023)
Structural basis of the interaction between BCL9-Pygo and LDB-SSBP complexes in assembling the Wnt enhanceosome.
Nat Commun 14(1): 3702