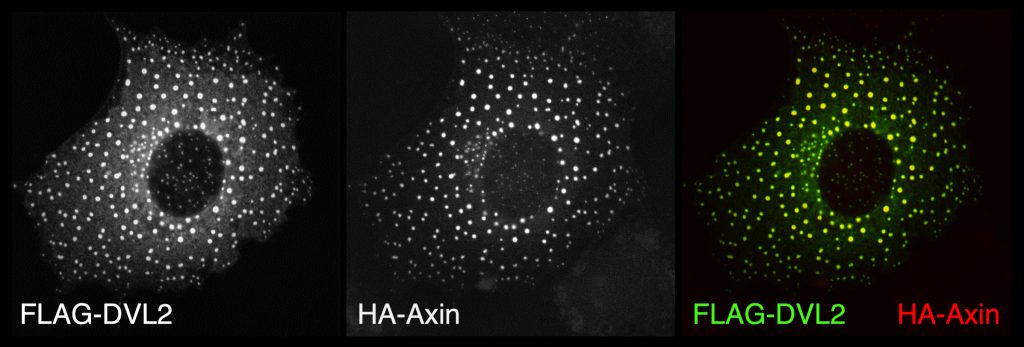
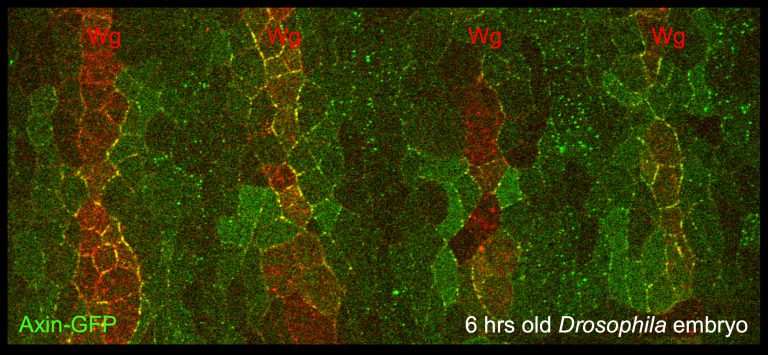
Dishevelled was known to be associated with the plasma membrane, transducing Wnt signals to all known Wnt effectors. This involves Dishevelled pivoting between Armadillo/beta-catenin and non-canonical signalling branches including those determining planar polarity. Using confocal microscopy, we discovered an essential function of Dishevelled in translocating the Axin degradasome from the cytoplasm to the cell surface near Wingless signalling sources in the epidermis of Drosophila embryos. Glycogen synthase kinase 3 (GSK3) is swept along as an Axin-associated component of the degradasome and reinforces this translocation (via phosphorylating specific motifs in the intracellular tail of the LRP5/6 co-receptors, as shown by Xi He and colleagues). A series of subsequent experiments led to the concept of the Wnt signalosome, a dynamic signalling platform assembled at the Wnt receptor complex. This involves dynamic head-to-tail polymerisation of its Dishevelled hub, which thus attains a high binding avidity for low-affinity Wnt signalling effectors such as Axin itself.
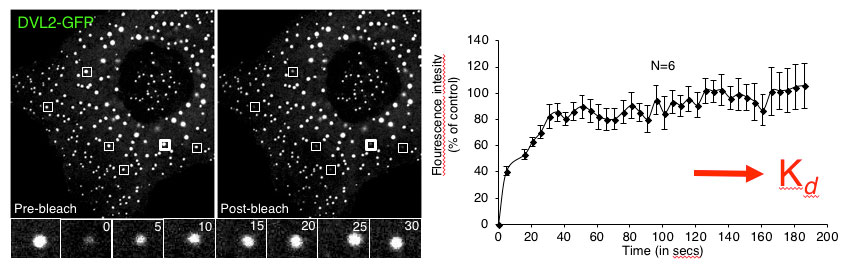
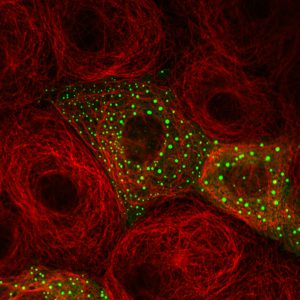
All three vertebrate Dishevelled orthologues (DVL1-3) form cytoplasmic puncta upon overexpression in various mammalian cells and tissues, thereby triggering Wnt-independent beta-catenin signalling. These puncta turned out to be highly dynamic protein assemblies without membrane components, and Fluorescence Recovery After Photobleaching (FRAP) experiments of individual DVL2-GFP puncta revealed that the punctate protein exchanges rapidly (half-time ~15 seconds) with the soluble monomeric pool of DVL2-GFP. If observed over 2-3 days, DVL2-GFP puncta grow and coalesce into huge blobs resembling ‘lipid droplets’ which eventually cause cell death, suggesting that the cellular levels of Dishevelled are limited normally by protein turnover.
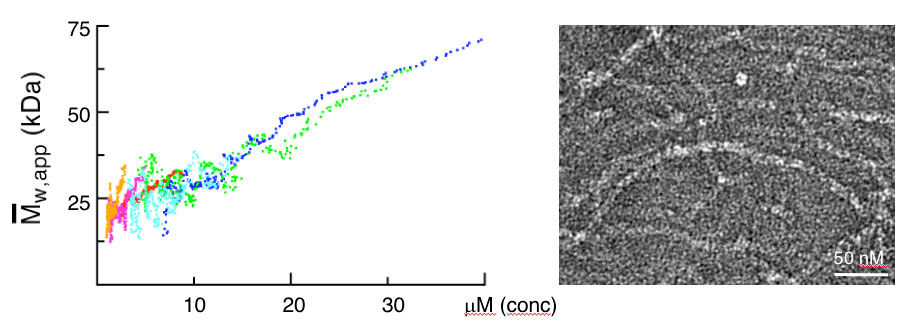
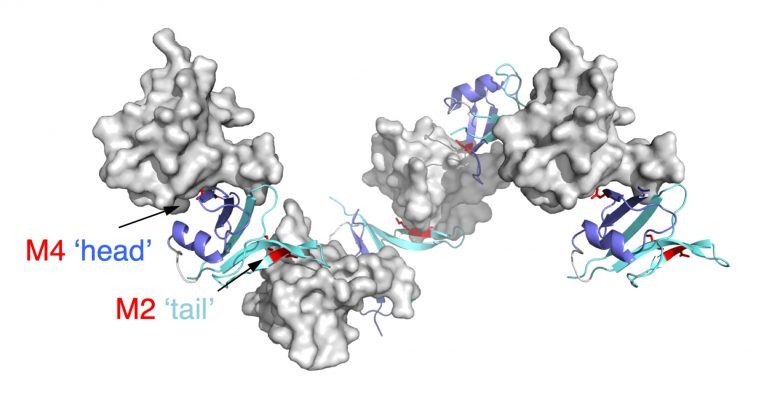
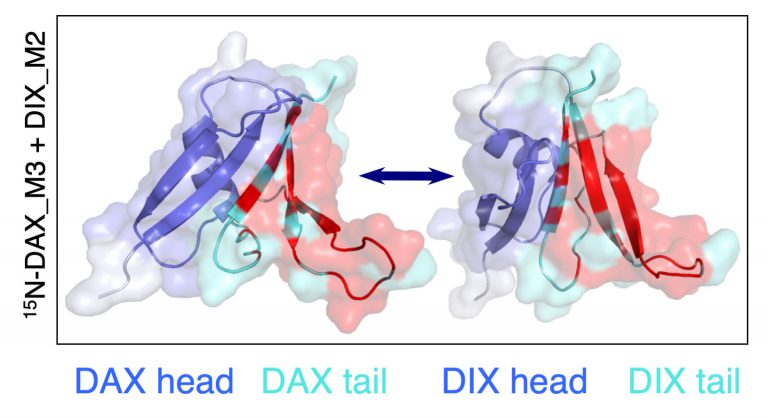
Importantly, we identified point mutations (M2-4) in the DIX domain of DVL2 that are required for signalosome assembly and signalling activity in vivo and, crucially, for self-association of purified DIX domain in vitro. These mutations allowed us to discover that the DIX domain undergoes dynamic head-to-tail polymerisation. Short reversible DIX domain oligomers can be detected by equilibrium ultracentrifugation, and DIX domain filaments can also be visualised by electron microscopy. Furthermore, purified DIX domain formed helical filaments upon crystallisation, and its structure revealed that the residues mutated in the signalling-defective M2-4 mutants are located in the DIX-DIX interface and engage in key DIX-DIX interactions. We concluded that Dishevelled is a hub protein that assembles a Wnt signalosome by head-to-tail polymerisation via its DIX domain to increasing the local concentration of Dishevelled effector binding sites. Consequently, polymerised Dishevelled attains a high binding avidity for low-affinity effectors such as Axin (the scaffold of the beta-catenin destruction complex, also known as ‘degradasome’), which it cannot bind at its normal physiological (sub-micromolar) cellular concentration.
It was known from Feng Cong’s work that Dishevelled binds constitutively to the intracellular face of the Frizzled receptor and mediates its turnover in the absence of Wnt. Binding of Wnt to the extracellular face of Frizzled triggers polymerisation of Dishevelled via its DIX domain, and co-polymerisation between this domain with that of Axin (called DAX) via a heterotypic DIX-DAX interaction. Thus, Dishevelled recruits the Axin degradasome to the Wnt receptor complex at the plasma membrane, which comprises Frizzled and its co-receptor LRP5/6 (which also binds to some of the Wnts). Furthermore, polymerised Dishevelled recruits casein kinase 1 (CK1) via its PDZ domain, thereby promoting the phosphorylation of specific motifs in the cytoplasmic tail of LRP5/6. Consequently, these phospho-motifs bind to the catalytic pocket of signalosome-associated GSK3 as competitive inhibitors (according to work by the Weis group), blocking its activity in the degradasome towards beta-catenin. This allows beta-catenin to accumulate, and to be captured in the nucleus by the BCL9 scaffold of the Wnt enhanceosome and loaded onto TCF/LEF, which in turns relieves the Groucho/TLE-dependent repression of linked Wnt target genes.
Signalosomes and degradasomes are three-dimensional molecular condensates, and so their constituent DIX and DAX polymers need to be cross-linked. In the Axin degradasome, APC and beta-catenin act as a cross-linkers of DAX filaments, and thus have essential functions in nucleating degradasome assembly. Furthermore, APC also prevents Axin from binding to Dishevelled at the plasma membrane in fly imaginal disc cells that are not exposed to Wingless stimulation – likely a mechanism to prevent inadvertent Wingless signal transduction.
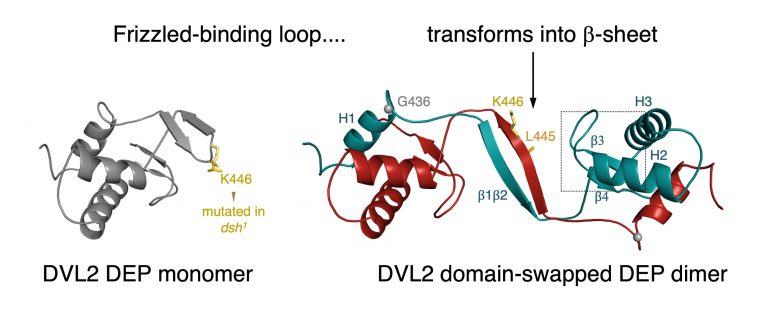
Cross-linking of DIX filaments in Wnt signalosomes is achieved by DEP-dependent dimerisation, which involves DEP domain swapping. This process blocks binding of Dishevelled to the Frizzled receptor, by masking the Frizzled-binding site located in an exposed loop of the monomeric DEP domain. This implies that the signalosomes gradually detach from the plasma membrane during chronic stimulation of cells by canonical Wnt signals. Therefore, DEP domain swapping must be attenuated during non-canonical Wnt signalling when the signalosomes need to be stably associated with Frizzled to orient the actin skeleton of incipient wing hairs, and other planar polarity signalling outputs.
One of factors limiting the cellular Dishevelled levels is Sudx/WWP2, a HECT E3 ubiquitin ligase that binds to Dishevelled and whose ligase activity towards Dishevelled is dis-inhibited by Dishevelled polymerisation. The latter also unlocks its ligase activity towards Notch, a functionally relevant substrate in fly imaginal discs and in ovarian cancer. In other words, Sudx/WWP2 downregulates signalling by polymerised Dishevelled and by Notch, thereby providing cross-talk between these two signalling pathways at the plasma membrane, within the Wnt signalosome.
Relevant references:
- Cliffe, A., Hamada, F., Bienz, M. (2003)
A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling.
Curr Biol 13(11): 960-6 - Schwarz-Romond, T., Merrifield, C., Nichols, B.J., Bienz, M. (2005)
The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles.
J Cell Sci 118(Pt 22): 5269-77 - Schwarz-Romond, T., Metcalfe, C., Bienz, M. (2007)
Dynamic recruitment of axin by Dishevelled protein assemblies.
J Cell Sci 120(Pt 14): 2402-12 - Schwarz-Romond, T., Fiedler, M., Shibata, N., Butler, P.J., Kikuchi, A., Higuchi, Y., Bienz, M. (2007)
The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization.
Nat Struct Mol Biol 14(6): 484-92 - Bilic, J., Huang, Y.L., Davidson, G., Zimmermann, T., Cruciat, C.M., Bienz, M., Niehrs, C. (2007)
Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation.
Science 316(5831): 1619-22 - Metcalfe, C., et al. (2010)
Dvl2 promotes intestinal length and neoplasia in the ApcMin mouse model for colorectal cancer.
Cancer Res 70(16): 6629-38 - Metcalfe, C., Mendoza-Topaz, C., Mieszczanek, J., Bienz, M. (2010)
Stability elements in the LRP6 cytoplasmic tail confer efficient signalling upon DIX-dependent polymerization.
J Cell Sci 123(Pt 9): 1588-99 - Metcalfe, C., Bienz, M. (2011)
Inhibition of GSK3 by Wnt signalling--two contrasting models.
J Cell Sci 124(Pt 21): 3537-44 - Fiedler, M., Mendoza-Topaz, C., Rutherford, T.J., Mieszczanek, J., Bienz, M. (2011)
Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating β-catenin.
Proc Natl Acad Sci U S A 108(5): 1937-42 - Bienz, M. (2014)
Signalosome assembly by domains undergoing dynamic head-to-tail polymerization.
Trends Biochem Sci 39(10): 487-95 - Mund, T., Graeb, M., Mieszczanek, J., Gammons, M., Pelham, H.R., Bienz, M. (2015)
Disinhibition of the HECT E3 ubiquitin ligase WWP2 by polymerized Dishevelled.
Open Biol 5(12): 150185 - Madrzak, J., Fiedler, M., Johnson, C.M., Ewan, R., Knebel, A., Bienz, M., Chin, J.W. (2015)
Ubiquitination of the Dishevelled DIX domain blocks its head-to-tail polymerization.
Nat Commun 6: 6718 - Gammons, M.V., Rutherford, T.J., Steinhart, Z., Angers, S., Bienz, M. (2016)
Essential role of the Dishevelled DEP domain in a Wnt-dependent human-cell-based complementation assay.
J Cell Sci 129(20): 3892-3902 - Gammons, M.V., Renko, M., Johnson, C.M., Rutherford, T.J., Bienz, M. (2016)
Wnt Signalosome Assembly by DEP Domain Swapping of Dishevelled.
Mol Cell 64(1): 92-104 - Gammons, M., Bienz, M. (2018)
Multiprotein complexes governing Wnt signal transduction.
Curr Opin Cell Biol 51: 42-49 - Yamanishi, K., Fiedler, M., Terawaki, S.I., Higuchi, Y., Bienz, M., Shibata, N. (2019)
A direct heterotypic interaction between the DIX domains of Dishevelled and Axin mediates signaling to β-catenin.
Sci Signal 12(611) - Gammons, M.V., Renko, M., Flack, J.E., Mieszczanek, J., Bienz, M. (2020)
Feedback control of Wnt signaling based on ultrastable histidine cluster co-aggregation between Naked/NKD and Axin.
Elife 9 - Bienz, M. (2020)
Head-to-Tail Polymerization in the Assembly of Biomolecular Condensates.
Cell 182(4): 799-811 - Krishnan, A., et al. (2020)
Proteogenomics analysis unveils a TFG-RET gene fusion and druggable targets in papillary thyroid carcinomas.
Nat Commun 11(1): 2056 - Kan, W., et al. (2020)
Limited dishevelled/Axin oligomerization determines efficiency of Wnt/β-catenin signal transduction.
Elife 9 - Beitia, G.J., Rutherford, T.J., Freund, S.M.V., Pelham, H.R., Bienz, M., Gammons, M.V. (2021)
Regulation of Dishevelled DEP domain swapping by conserved phosphorylation sites.
Proc Natl Acad Sci U S A 118(26) - Mieszczanek, J., Strutt, H., Rutherford, T.J., Strutt, D., Bienz, M., Gammons, M.V. (2022)
Selective function of the PDZ domain of Dishevelled in noncanonical Wnt signalling.
J Cell Sci 135(11)
- Gammons M, Franco-Echevarría E, Li T, Rutherford TJ, Renko M, Batters C & Bienz M (2024)
Wnt-driven inhibition of GSK3 relies on LRP6 binding to the Axin-GSK3 complex and AP-2 clathrin adaptor
In preparation