The macromolecular determinants of nuclear shape
Our primary objective is the investigation of the structural organisation and conformational adaptations (molecular plasticity) of protein complexes ruling nuclear architecture and shape in situ in physiological or pathological conditions. High-resolution details will help us to understand the molecular basis underlying nuclear remodelling. Mutations and alterations in these protein complexes are frequently associated with disease states (ageing, cancer, developmental disorders) and nuclear deformations have been shown to impact nuclear transport and gene expression.
To investigate the universal principles determining nuclear architecture and shape, we have been using two different human model systems where the nucleus is being drastically remodelled: sperm cells and somatic migratory cells. We aim to elucidate the higher-order nucleus-cytoskeleton architectures required for differentiation and cell migration across various human cell types, including cancer cells with enhanced migration capabilities. We employ in-cell structural biology techniques, integrated with complementary structural and cell biology methods.
Some questions we are interested:
- Which are the macromolecular determinants of nuclear shape / function?
- How is force transmitted from the cytoskeleton to the nucleus?
- How can we streamline high-resolution in situ structural determination?
- Which are the macromolecular bases underlying the enhanced migratory capabilities of cancer cells?
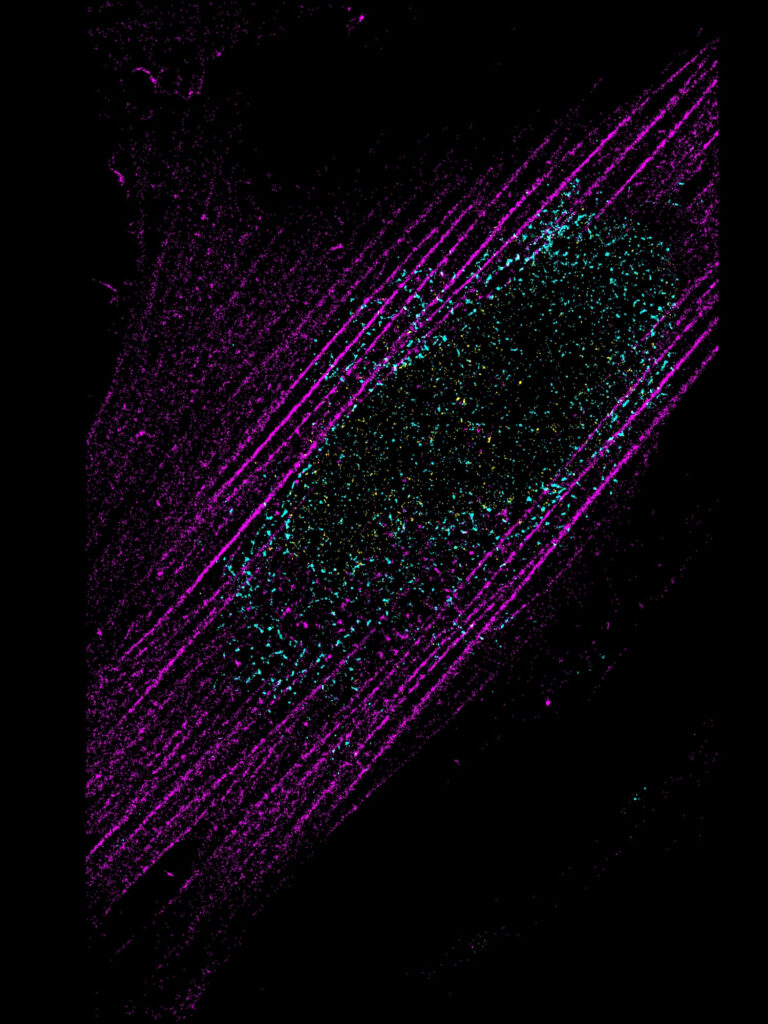
nucleo-cytoskeleton interaction in fibroblasts