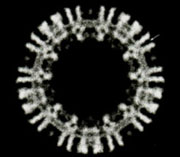
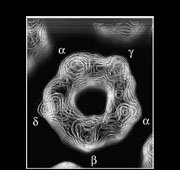
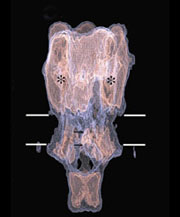
Structures determined from the tubes give a three-dimensional picture of the ACh receptor in its native membrane setting, in the closed-channel form. The whole pentamer is ~160Å long and up to ~80Å in diameter. It has a central ion path that is ~20Å dia. in the extracellular portion, narrow across the membrane, and opens up into the cell interior through five side-windows.
A series of technical developments allowed detail to be elucidated at ever increasing resolution. Most important were: the low-dose imaging of tubes in ice over holes in the carbon support, to ensure all surfaces were exposed equally to a natural ionic environment; the correction of tube distortions (bending, changing twist etc.), which are always present in the images; use of a highly stable electron microscope, incorporating a liquid helium-cooled stage.
Key publications:
Toyoshima, C. and Unwin, N. Ion channel of acetylcholine receptor reconstructed from images of postsynaptic membranes. Nature 336, 247-250 (1988). (pdf)
Toyoshima, C. and Unwin, N. Three-dimensional structure of the acetylcholine receptor by cryoelectron microscopy and helical image reconstruction. J. Cell Biol. 111, 2623-2635 (1990). (pdf)
Unwin, N. Nicotinic acetylcholine receptor at 9Å resolution. J. Mol. Biol. 229, 1101-1124 (1993). (pdf)
Beroukhim, R. and Unwin, N. Distortion correction of tubular crystals: improvements in the acetylcholine receptor structure. Ultramicrosc. 70, 57-81 (1997). (pdf)
Miyazawa, A., Fujiyoshi, Y., Stowell, M. and Unwin, N. Nicotinic acetylcholine receptor at 4.6Å resolution: tranverse tunnels in the channel wall. J. Mol. Biol. 288, 765-786 (1999). (pdf)