
Michael Rossmann, Hanley Distinguished Professor of Biological Sciences at Purdue University and former scientific staff at the LMB, where he worked with Max Perutz on the structure of haemoglobin, has died on 14th May 2019 at the age of 88. Michael was a very gifted crystallographer whose main strength was in mathematics and computer programming. At Purdue, Michael’s central focus of research was the determination of the three-dimensional, atomic resolution structure of viruses and how viruses interact with their environment. Michael led a team of researchers who were the first to map the structure of the human common cold virus to an atomic level. He discovered the Rossmann fold protein motif, initially in the enzyme lactate dehydrogenase and then found in hundreds of other proteins.
Michael was born on 30th July 1930 in Frankfurt, Germany. He attended schools in Germany and Holland before moving to England in 1939, where he joined the Friends’ School in Saffron Walden. Here, he had his first contact with crystallography: Kathleen Lonsdale, who had worked with W H Bragg and had her own crystallography group at University College London, was one of the school’s governors. Michael undertook degrees in mathematics and physics at University College London and then obtained a PhD, ‘A study of some organic crystal structures’ from the University of Glasgow, under J M Robertson.
In 1956, Michael joined the lab of Bill Lipscomb, at the University of Minnesota, USA, and worked on the structure of terpenoids, plant products with about 30 non-hydrogen atoms. He wrote computer programmes, in machine code, for analysing structures on the early UNIVAC 1103 computer. At a talk by Dorothy Hodgkin, at the International Crystallography Meeting in Montreal, he heard about the work on the structure of proteins being undertaken in Cambridge, England. Michael thought the scale of the problem sounded both attractive and challenging, so he wrote to Max Perutz to enquire about joining his group. An enthusiastic Max offered Michael a job and in 1958, Michael joined the MRC Unit, in the tiny hut outside the main Cavendish Laboratory building.

Michael arrived at the LMB at a time of great activity, when the work on determining the structure of haemoglobin was reaching its peak. Michael’s experience of mathematical methods and computer programmes was harnessed to help determine the relative y-axis coordinates of the heavy atom sites for the different heavy-atom derivatives of haemoglobin, from the data collected by Max and his research assistant, Ann Cullis. The haemoglobin crystals were monoclinic (one two-fold axis) and so there were no symmetry-determined special points along one axis in the unit cell that could define an origin in that direction. Dismissing proposals from Max, Francis Crick and others, Michael predicted that a Patterson calculated with the squared difference Fourier coefficients would have negative peaks representing the vectors between the heavy atoms in compound 1 and the heavy atoms in compound 2. The Lab had access to the new EDSAC2 computer, housed in the nearby Mathematics Laboratory, and during the designated Monday night slots, Michael began to compute his type of three-dimensional ‘correlation’ function. Michael quickly saw that the results were very easy to interpret – he had developed a method for determining the relative position of heavy atoms that left no doubt as to their positions. Subsequently, Michael developed a least-squares procedure based on this function to refine the heavy atom parameters. These provided the basis of the phase determination for the 5.5Å resolution map of haemoglobin which was calculated in 1959. Michael’s role was pivotal in discovering the structure of haemoglobin. He also, along with David Blow, developed a number of highly original mathematical methods to use the information present due to non-crystallographic symmetry, now known as molecular replacement, in protein crystallography. On his time in Cambridge, Michael later commented, “I was sad to leave Cambridge, where so much had happened in a short 6 years, but the foundations of my subsequent work had been laid”.
Michael moved to the Department of Biological Sciences, Purdue University, USA in 1964. His work there, initially on enzymes, quickly moved to focus on investigating the structure of viruses and their proteins. In 1973 he published the description of a nucleotide binding motif found in enzymes or kinases, which was named the Rossmann fold. In 1985, Michael’s group published their map of the structure of the human common cold virus to an atomic level. During the 1990s, Michael showed that the dengue virus changes shape when it enters its host, indicating that vaccines should mimic a virus’s body shape. Michael’s discoveries have suggested a shared origin for plant and animal viruses.
Michael stayed at Purdue for the rest of his career, becoming the Hanley Distinguished Professor of Biological Sciences in 1978. He was a member of the National Academy of Sciences, a Fellow of the American Academy of Arts and Sciences, an elected Fellow of the American Academy of Microbiology and Fellow of the American Association for the Advancement of Science. He was elected a Foreign Member of the Royal Society in 1996.
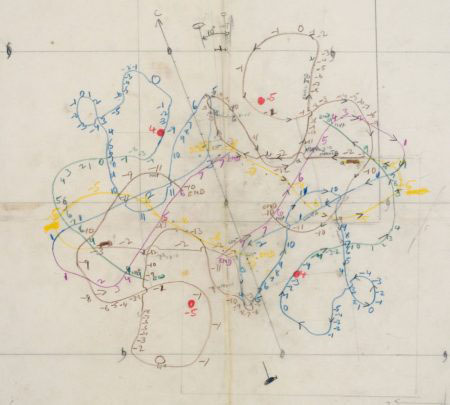
Michael remained a good friend of the LMB, returning as a sabbatical visitor to Richard Henderson’s group in 1980. Michael was one of the earliest X-ray crystallographers to broaden his interests to include electron microscopy and built up a very powerful critical mass of structural biology faculty at Purdue. He attended many LMB events and also donated several items to the LMB Archive, including a set of contour maps of haemoglobin, hand drawn and interpreted by Michael in 1959.
Richard Henderson commented: “Michael was a wonderful scientist, whose highly intense intellect was always sharply focussed on analysing, understanding and solving any problem involving crystallography, protein structure, drug design or function. He had immense energy which he always directed towards his main goal in a highly efficient manner. His research was at the scientific frontier for over seven decades”.