Fluctuating pH levels accompanying synaptic activity alters AMPAR N-terminal domain conformation via proton sensing, resulting in reduced receptor function and increased diffusion at the synapse
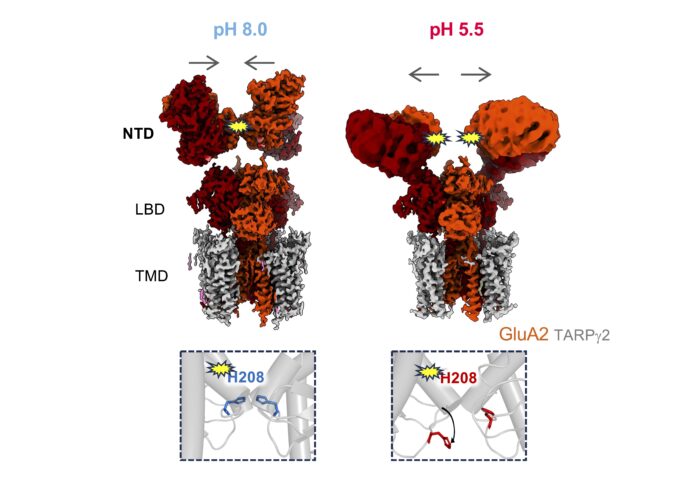
Inset: all-atom MD simulation at pH 8.0 (left) and at pH 5.5 (right). Protonation of His208 at pH 5.5 leads to interface destabilisation due to charge repulsion.
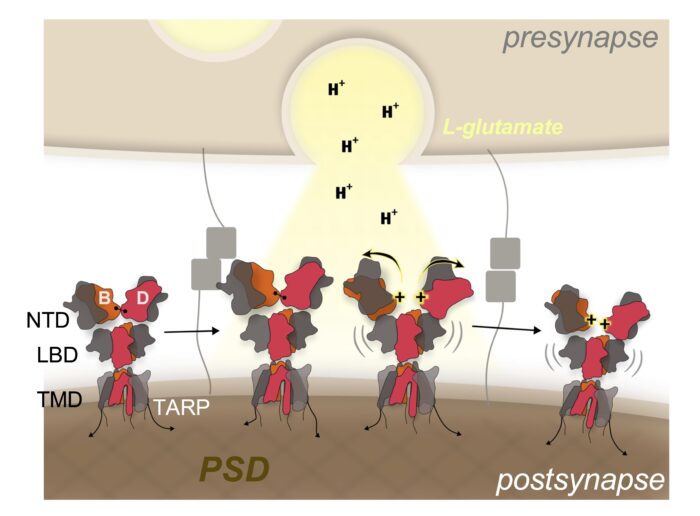
Within the brain, the release of acidic neurotransmitter vesicles results in pH fluctuations in active neuronal synapses. Prolonged acidosis of the synaptic cleft is associated with pathophysiological conditions such as ischemic strokes, neurodegenerative diseases, and seizures. Protons profoundly impact the function of various synaptic proteins, including AMPA receptors (AMPARs), which mediate fast, excitatory synaptic transmissions when activated by the neurotransmitter, L-glutamate. Low pH reduces AMPAR signalling, but the mechanism behind proton modulation of AMPARs has remained unknown. Now Ingo Greger’s group, in the LMB’s Neurobiology Division, have shown how protons trigger a rearrangement of the N-terminal domain (NTD) tier within AMPARs, thereby impacting receptor diffusion and synaptic transmission in response to fluctuating pH levels.
AMPARs are ion channel tetramers comprised of four core subunits – GluA1 to GluA4 – which assemble in various combinations with supporting, auxiliary subunits. The GluA2 subunit dominates AMPAR function in the forebrain. Its extracellular NTD plays a vital role in anchoring the AMPARs in the post-synaptic membrane beneath presynaptic neurotransmitter release sites, ensuring reliable synaptic transmission. Changes in the number of AMPARs anchored in this optimal location impacts synaptic strength and plasticity (the ability of synapse to strengthen or weaken over time), which underlies learning and memory within the brain.
Building on their previous studies of the GluA1/2 AMPA receptor, the team used a combination of techniques to unpick how protons tune AMPAR-mediated synaptic transmission. Firstly, Josip Ivica utilised patch-clamp electrophysiology to record the currents of both wild type and mutant AMPARs, revealing that the GluA2 NTD selectively tunes receptor responsiveness under low pH conditions.
Nejc Kejzar followed this with molecular dynamics simulations (MD) of the GluA2 NTD under various pH conditions. This led to the discovery of a proton sensor, histidine 208 (His208), embedded at an interface between adjacent NTDs. Under low pH conditions protonation of His208 triggers a rearrangement between the adjacent NTDs.
To investigate this structural change further, the team worked with Terunaga Nakagawa, a sabbatical visitor to the LMB and Professor at the Vanderbilt Brain Institute, US, to use electron cryo-microscopy to map the structures of GLuA2 at low and high pH levels. At pH 8.0, the NTD tier forms the canonical compact and upright arrangement. However, under pH 5.5, it transforms to a splayed, open arrangement.
Finally, Imogen Stockwell and Viktor Kuchtiak used fluorescence recovery after photobleaching (FRAP) of hippocampal neurons to image synaptic AMPARs under varying pH levels. This showed an increase in receptor diffusion of splayed NTDs in comparison to compact NTDs.
Taken together, these results demonstrate that the release of acidic glutamate leads to a proton-triggered conformational change in the NTD of the GluA2 subunit in AMPARs from a compact to a splayed, open arrangement. This releases the receptors from their synaptic anchor points, which then diffuse away from active sites in the postsynaptic membrane. Such a mechanism has capacity to tune the strength of synaptic transmission and plasticity, which underlies learning and memory in the brain.
Learning and memory formation processes in the brain are not yet well understood. This research into the mechanisms behind AMPAR synaptic organisation present an advancement of knowledge of how synapses store memories. Further investigation could reveal how disruptions to this mechanism contributes to neurodegenerative diseases in which memory and recollection are compromised. Furthermore, as the proton concentration in the synaptic cleft is known to increase during conditions including stroke, further studies could address whether AMPAR diffusion is affected and linked to symptoms,
This work was funded by UKRI MRC, UKRI BBSRC, the Wellcome Trust and the National Institute of Health.
Further references
Proton-triggered rearrangement of the AMPA receptor N-terminal domains impacts receptor kinetics and synaptic localisation. Ivica, I., Kejzar,N., Ho, H., Stockwell, I., Kuchtiak, V., Scrutton, A.M., Nakagawa, T., Greger, I.H. Nature Structural & Molecular Biology
Ingo’s group page
Terunaga Nakagawa’s page
Nature Research Briefing
Vanderbilt University article
Previous Insight on Research articles
First Cryo-EM structures of homomeric GluA1 AMPA glutamate receptor reveals functional roles for N-terminal domains
New structures show how auxiliary subunits modulate hippocampal AMPA receptor neurotransmission
Architecture of a prominent neurotransmitter receptor involved in memory formation and learning revealed