A new factor has been found to help nascent secretory and membrane proteins quickly access the protein translocation machinery when they arrive at the endoplasmic reticulum
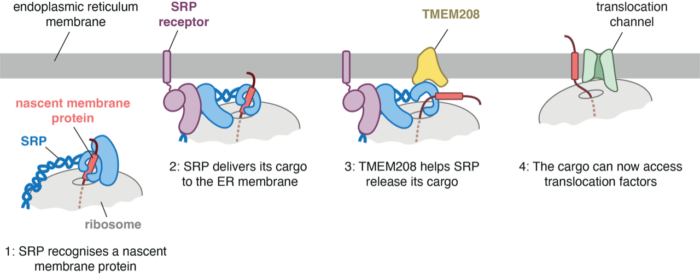
Around one-third of all genes code for proteins that need to be secreted or embedded in a cell membrane. In eukaryotic cells, these proteins are synthesized at the surface of the endoplasmic reticulum (ER), a vast membrane network which contains the machinery for protein folding, modification, and assembly. Delivery of secreted and membrane proteins to the ER surface occurs as they start emerging from the ribosomes tasked with their synthesis. A factor called signal recognition particle (SRP) recognises and binds tightly to a hydrophobic segment, typically located near the beginning of nascent secretory and membrane proteins. SRP delivers these nascent proteins to a receptor located at the ER. But how SRP lets go of its cargo selectively at the ER has been unclear. New research from Huping Wang in Manu Hegde’s group in the LMB’s Cell Biology Division shows that cells contain an ER-resident protein, called TMEM208, that helps SRP release its cargo, which can then engage factors for translocation into or across the ER membrane.
Earlier work from Manu’s group discovered new factors for the insertion and folding of membrane proteins. Previous genetic analyses had suggested that TMEM208 might also somehow be involved in making membrane proteins, prompting Huping’s interest. She first showed that cells lacking TMEM208 grew poorly and indeed displayed problems in making most multipass membrane proteins (those that span the membrane multiple times, such as transporters and channels). Huping then set out to determine the molecular basis of this phenotype. She recapitulated multipass membrane protein biogenesis in a test-tube using cell extracts. She used this simplified system to find the step where membrane proteins failed to be made correctly in the absence of TMEM208. It turned out to be at the very first step before membrane proteins even began insertion into the ER membrane.
Tracking down this defect further, she discovered that TMEM208 interacts with SRP specifically in the region used by SRP to bind its cargo. Using biochemical assays, she could show that without TMEM208, SRP was slow in releasing its cargo after the SRP-cargo complex arrives at the ER. The magnitude and consequence of this delay varied depending on the cargo. Cargos that were especially hydrophobic, including many membrane proteins and some secretory proteins, were bound especially tightly to SRP and needed help from TMEM208 for release. Furthermore, multipass membrane proteins were especially sensitive to a delay in release from SRP. These proteins need to be carefully stitched into the membrane, and a delay in release from SRP caused too much of the protein to be synthesized before membrane protein insertion could begin.
TMEM208 therefore ensures that there is an orderly handover of secretory and membrane protein cargoes from SRP to the translocation factors needed for their biogenesis. A delay in this critical delivery step risks cargoes getting mislocalised in the cell, leading to their degradation. This is analogous to package deliveries at home – if nobody is there to receive them, they can be mislaid, lost, or stolen. In cells, orderly and efficient deliveries of secretory and membrane proteins to the ER are crucial for cell health. Even the modest inefficiency caused by loss of TMEM208 reduces a cell’s capacity to successfully make secretory and membrane proteins, while also increasing the number of misfolded proteins made in these cells. Both consequences are detrimental, with protein misfolding being associated with numerous diseases ranging from cystic fibrosis to neurodegeneration.
This work was funded by UKRI MRC and EMBO.
Further references
Identification of a factor that accelerates substrate release from SRP. Wang, H., and Hegde, R.S. Science
Manu’s group page
Previous Insight on Research articles
Pathway for making multipass membrane proteins elucidated
Discovery of a chaperone for membrane proteins