Time-resolved study of in vitro assembly of tau identifies the formation of disease-specific intermediate amyloid filaments

The abnormal assembly of a small number of proteins into amyloid filaments defines more than 95% of cases of human neurodegenerative disease. Previous electron cryo-microscopy (cryo-EM) studies at the LMB, led by Sjors Scheres, Benjamin Ryskeldi-Falcon and Michel Goedert, revealed that different neurodegenerative diseases are characterised by specific amyloid filament folds. However, the molecular mechanisms underlying the formation of these folds remained unknown.
It is thought that intermediate filament species with differing structures form during the pathways leading to mature amyloid filaments. Moreover, these intermediate species are believed to play a role in amyloid toxicity, so they are important therapeutic targets. However, the transient nature of such filament species makes them difficult to study and, until recently, attempts to assemble amyloids in vitro yielded filaments with cores that were different from those in brain.
Now, for the first time, the groups of Sjors Scheres and Michel Goedert, from the LMB’s Structural Studies and Neurobiology Divisions, report the different structures of disease-specific intermediate filaments on the pathways to mature Alzheimer or chronic traumatic encephalopathy (CTE) tau filaments.
Sofia Lövestam, joint postdoc in the Scheres and Goedert groups, recently identified conditions under which recombinant truncated tau (amino acids 297-391, in the numbering of the 441 amino acid human brain tau isoform) assembles into the same amyloid filaments as those extracted from the brains of individuals with Alzheimer’s disease or CTE. Building on this work, Sofia characterised the filaments that form over time. By taking small samples out of the reactions and looking at them by cryo-EM, Sofia and colleagues were able to solve structures of the intermediate filaments that form during the events leading to the formation of Alzheimer and CTE folds. In total, they solved 163 cryo-EM structures yielding 44 unique structures.
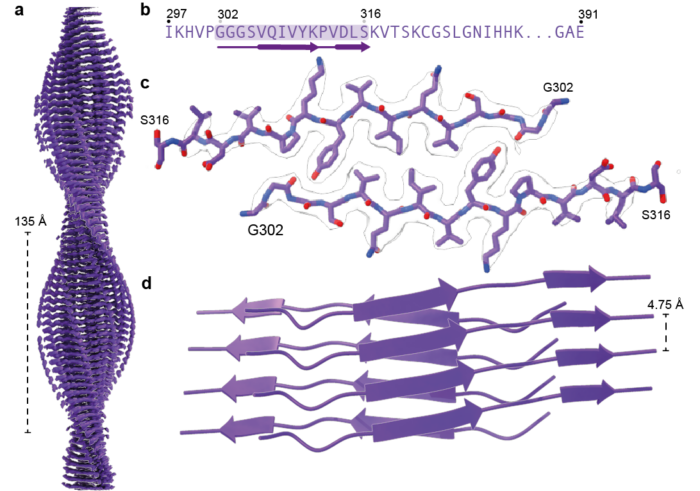
Interestingly, under both reaction conditions, the same initial structure was observed, termed the First Intermediate Amyloid (FIA). The FIA forms after two hours and comprises only 15 amino acids of two copies of tau on each layer of the amyloids, making it one of the smallest filaments ever solved by cryo-EM. By three hours, the FIA has disappeared and instead many different filament types are present, which also disappear and turn into other types. After twelve hours, most tau filaments have the Alzheimer or CTE fold.
Studying the differences between structures allowed the team to propose models for how the filaments “mature” over time. The findings obtained in this study suggest that formation of disease-specific amyloid filaments begins with tau monomers nucleating into FIAs, which grow and turn into mature filaments via multiple secondary nucleation events, that result in the formation of different intermediate amyloids.
These findings increase our understanding of how tau filaments form, which may lead to the development of therapeutic interventions to prevent their formation in the brains of individuals with Alzheimer’s disease or CTE.
This work was funded by UKRI MRC and a Marshall scholarship.
Further references
Disease-specific tau filaments assemble via polymorphic intermediates. Lövestam, S., Li, D., Wagstaff, JL., Kotecha, A., Kimanius, D., McLaughlin, S.H., Murzin, AG., Freund, SMV., Goedert, M., Scheres, SHW. Nature
Sjors’ group page
Michel’s group page
The tale of tangled tau in Alzheimer’s disease
Assembly of recombinant tau into filaments identical to those of Alzheimer’s disease and chronic traumatic encephalopathy eLife
Molecular pathology of neurodegenerative diseases by cryo-EM of amyloids Nature
Transient Filament Yields AD and CTE Tau Fibrils Alzforum
Amyloid study shows formation pathway of tau filaments C&EN
Previous Insight on Research articles
Identical structures of α-synuclein filaments from Parkinson’s disease and dementia with Lewy bodies
TMEM106B filaments form in an age-dependent manner in human brains
Atomic structures of Aβ42 filaments from the brains of individuals with Alzheimer’s disease and other neurodegenerative conditions