New structures of tau filaments from corticobasal degeneration are the first to be revealed for the most common category of tauopathies after Alzheimer’s disease
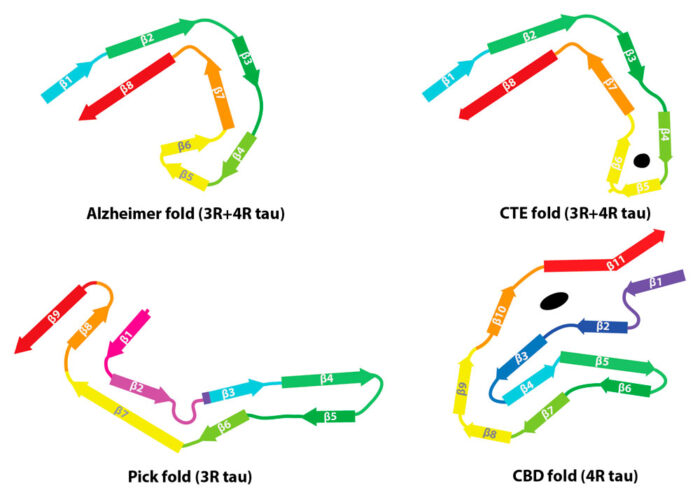
Corticobasal degeneration (CBD) is a neurodegenerative disease involving the cerebral cortex and basal ganglia. CBD belongs to a family of diseases called tauopathies in which the protein tau forms abnormal filaments. Sjors Scheres’ and Michel Goedert’s groups have now solved the first structures of tau filaments from the cerebral cortex of patients with CBD. Importantly, they have also shown that these structures are different from those they previously solved for tau assemblies from Alzheimer’s disease, Pick’s disease, and chronic traumatic encephalopathy (CTE).
Tau protein is found in six different versions, or isoforms, that are produced by alternative mRNA splicing. These isoforms differ in the presence or absence of a sequence of amino acids near the start of the protein chain and the inclusion or exclusion of a section of the microtubule binding domain nearer the end of the protein. The microtubule binding domain is formed of three or four repeating sequences of amino acids, depending on whether this section is excluded or included. Inclusion of this repeat in three of the tau isoforms results in versions with four repeats (known as 4R), whereas exclusion in the other three isoforms leaves those with three repeats (3R).
There are more than 20 different tauopathies, with Alzheimer’s disease being the most common. Tauopathies can be categorised based on which isoforms of tau are incorporated into the filaments: 3R only, 4R only, or 3R and 4R. After Alzheimer’s disease, 4R tauopathies are the most common form of this family of diseases. Sjors’ group in the LMB’s Structural Studies Division and Michel’s group in the Neurobiology Division had previously solved the structures of tau filaments from two diseases incorporating 3R and 4R isoforms of tau, Alzheimer’s disease and CTE, and from one 3R tauopathy, Pick’s disease. They have now solved the first structures of tau filaments from a 4R tauopathy, CBD.
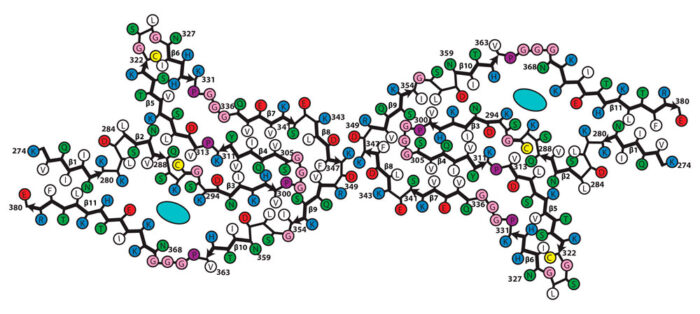
Working with brain tissue preparations from three CBD patients, provided and characterised by Masato Hasegawa from the Tokyo Metropolitan Institute of Medical Science, and Bernardino Ghetti from Indiana University, Wenjuan Zhang, a researcher in Sjors’ group, solved the structures of tau filaments using electron cryo-microscopy. She found that the three individuals each had the same filament structure and that this structure was different to those previously found for Alzheimer’s disease, Pick’s disease, and CTE. These new structures also reveal the presence of additional molecules in a cavity within the filament. These molecules have not yet been identified, but it is possible that they are involved in filament formation.
Every tauopathy for which a tau filament structure has been solved has a unique disease-specific fold. Structures of filaments from different tauopathies may be useful for the development of tracer compounds that are specific for the folds of tau that can be used for diagnosis in live patients. Understanding how and why tau assembles into different disease-specific structures will also be beneficial for the development of new therapies. It might, for example, be possible to design drugs that stop filaments forming in order to stop the progression of neurodegeneration.
The work was funded by the MRC, European Union, Japan Agency for Science and Technology, Japan Agency for Medical Research and Development, US National Institutes of Health, and the Department of Pathology and Laboratory Medicine at Indiana University School of Medicine.
Further references:
Novel tau filament fold in corticobasal degeneration. Zhang, W., Tarutani, A., Newell, KL., Murzin, AG., Matsubara, T., Falcon, B., Vidal, R., Garringer, HJ., Shi, Y., Ikeuchi, T., Murayama, S., Ghetti, B., Hasegawa, M., Goedert, M., Scheres, SHW. Nature (Epub ahead of print)
Sjors’ group page
Michel’s group page