Ageing is a growing problem for society, particularly because of the associated increased risk of developing disease. Understanding how we might be able to live healthier for longer is a key goal of medical research. The nematode worm C. elegans is a commonly used model for studying the changes that take place as animals age. Rebecca Taylor’s group in the LMB’s Neurobiology Division, in collaboration with researchers from the Epigenetics Programme at the Babraham Institute, have now identified how expression of one particular stress response gene enhances longevity in worms, which could have implications for neurodegenerative diseases such as Alzheimer’s disease.
Rebecca had previously identified that expression of a stress response gene, XBP-1, in neurons could activate a stress response programme in the intestine of worms, increasing their longevity and making them more resistant to environmental stress. However, it was not clear how the longer lifespan of these animals was orchestrated. Soudabeh Imanikia, a researcher in Rebecca’s group, now investigated which genes were expressed at higher levels in the long-lived worms compared to normal worms. She found that genes associated with lysosomal function were more active in the intestine of these animals, suggesting a mechanism that might increase their longevity.
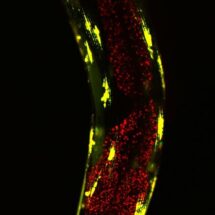
Lysosomes can act as a waste disposal system to digest and remove unwanted molecules, and this activity of lysosomes depends upon their acidity. By using a dye that is activated in acidic conditions, Rebecca’s group were able to show that the XBP-1-expressing long-lived worms had more acidic, and therefore more active intestinal lysosomes than normal worms. Interestingly, although ageing lysosomes normally show reduced acidity, the lysosomes of the long-lived worms stayed acidic. In addition, worms expressing proteins that cause neurogenerative diseases were more resistant to disease symptoms when XBP-1 was expressed in neurons, which required functional lysosomes, suggesting that the more active lysosomes in these animals may be better able to digest the toxic misfolded proteins that cause disease.
In a second publication, Rebecca’s group, in collaboration with Julian Griffin’s group at the University of Cambridge, found that one effect of activating lysosomes may be to change the balance of fats within cells, and that this contributes to longevity and resistance to the effects of misfolded disease-causing proteins. In particular, the team found an increased amount of the fatty acid oleic acid inside long-lived worms. Interestingly, supplementing the diet of normal worms with increased amounts of oleic acid increased their lifespan and helped their cells cope better with the presence of misfolded neurodegenerative disease-causing proteins.
Insights into how we might be able to live longer and healthier are of critical importance, particularly within the context of ageing-related neurodegenerative diseases such as Alzheimer’s disease. This finding that activation of one specific cellular compartment can confer healthy longevity and resistance to diseases caused by protein misfolding provides a potential future target for therapies. As oleic acid is commonly found in natural oils and foods, such as olive oil and avocados, this work may also strengthen the case for the importance of diet in long-term health.
The work was funded by the MRC.
Further references
Neuronal XBP-1 activates intestinal lysosomes to improve proteostasis in C. elegans. Imanikia, S., Özbey, NP., Krueger, C., Casanueva, MO., Taylor, RC. Current Biology [Epub ahead of print]
XBP-1 remodels lipid metabolism to extend longevity. Imanikia, S., Sheng, M., Castro, C., Griffin, J., Taylor, RC. Cell Reports [Epub ahead of print]
Rebecca’s group page
Babraham Institute Epigenetics Programme
Julian Griffin’s page