ATP-competitive inhibitors of the kinases of the integrated stress response (ISR) can unexpectedly activate the ISR by directly binding to and activating a sister ISR kinase
The integrated stress response (ISR) is a central controller of cell and organism fitness which reduces protein synthesis and reprogrammes gene expression to adapt to and survive challenges. Due to its central role in cell fitness, the ISR is an important therapeutic target in a range of diseases, from cancer to neurodegeneration. A number of ISR kinase inhibitors have been developed and used to dissect the role of ISR in cells and organisms as well as to evaluate the therapeutic potential of ISR inhibition in disease models. New research from Anne Bertolotti’s group in the LMB’s Neurobiology Division has discovered unexpected dose-dependent activation, rather than the intended inhibition, of ISR by its kinase inhibitors.
ISR is activated by phosphorylation of the translation initiation factor eIF2α. In humans the response is initiated by four eIF2α protein kinases, GCN2, HRI, PKR and PERK, which sense different stimuli. These protein kinases have been the focus of several large drug discovery programmes, and multiple ATP-competitive inhibitors have been developed. The drugs effectively inhibit their target kinases at nanomolar concentrations but are typically used at micromolar concentrations to ensure the target kinase is saturated, a standard protocol for kinase inhibition.
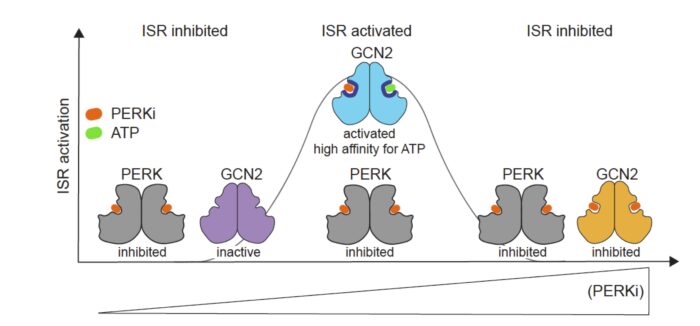
When using one such drug, GSK2656157, at the standard micromolar concentration to inhibit PERK, Maria Szaruga, a postdoc in Anne’s group, unexpectedly found that ISR was activated rather than inhibited. Further investigation revealed that whilst a range of PERK inhibitors did inhibit PERK in the nanomolar range, ISR was activated when the drugs were used in the standard micromolar range. The team discovered that this unanticipated activation occurred via the GCN2 kinase.
To unpick what was happening, the team reconstituted ISR activation using recombinant proteins and found that PERK inhibitors directly activate the GCN2 dimer by increasing its affinity for ATP.
At nanomolar concentrations, the ATP-competitive inhibitors bind only to their primary target PERK and inhibit ISR, as expected. The inhibitors saturate PERK at micromolar concentrations, but are also able to bind to the ATP-binding site of GCN2 with lower affinity. Thus, before saturating all GCN2 binding sites, the compounds occupy only one protomer of the GCN2 dimer and this in turn, Maria found, increases the affinity for ATP of the second GCN2 protomer leading to GCN2 activation. This off-target activation of GCN2 by low micromolar concentrations of PERK inhibitors, was also observed for PKR kinase inhibitors, and occurred in the absence of the stress that naturally activates the kinases, so bypassed the need for natural ligands to activate GCN2.
These findings are important because ISR kinase inhibitors have been used in over 200 pre-clinical studies during the past 10 years, but at the concentrations broadly used, these compounds activate ISR via GCN2. The mechanism identified here might also be relevant to other kinases, a group of proteins that are prevalent drug targets. It is anticipated that these findings will provide the basis to improve existing kinase drugs, for example by refining selectivity and therefore helping to reduce clinical side effects, as well as guide the design of the next generation of drugs targeting protein kinases.
This work was funded by UKRI MRC, the Wellcome Trust, Human Frontier Science Programme (HFSP) and European Molecular Biology Organization (EMBO).
Further references
Activation of the integrated stress response by inhibitors of its kinases Szaruga, M., Janssen, DA., de Miguel, C., Hodgson, G., Fatalska, A., Pitera, AP., Andreeva, A., Bertolotti, A. Nature Communications
Anne’s group page
Previous Insight on Research articles
Making the undruggable druggable: the first platform to discover selective phosphatase inhibitors
Uncovering the action of a selective holophosphatase inhibitor