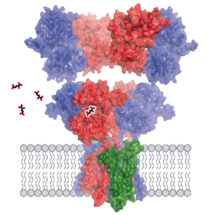
AMPA receptors are among the most commonly found receptor in the nervous system and play an important role during memory formation and learning. They are composed of four subunits with various possible combinations. Although AMPA receptors act predominantly as heteromeric complexes, structural studies to date have focused on assemblies made from four copies of the same subunit. Ingo Greger’s group in the LMB’s Neurobiology Division have now revealed a high resolution structure of the principal AMPA receptor complex found in the hippocampus, a part of the brain that is involved in memory formation.
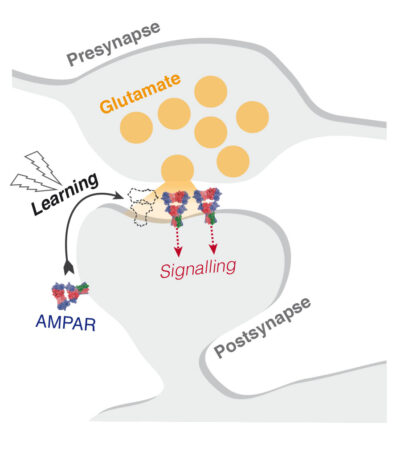
Communication between neurons at excitatory synapses in the brain involves release of the neurotransmitter glutamate (Glu) from the presynaptic neuron and detection of glutamate at the postsynaptic neuron by receptor ion channels. AMPA receptors are one of the main types of glutamate-responsive ion channels. Binding of glutamate opens the pore to allow sodium ions to flow into the postsynaptic neuron, which propagates a signal onward.
The most common AMPA receptor complex found in the hippocampus consists of two copies of the GluA1 subunit and two copies of GluA2. This GluA1/2 complex is selectively recruited to hippocampal synapses during synaptic strengthening processes involved in memory formation and learning. AMPA receptor function is diversified by association with auxiliary subunits, such as the TARP family, with TARP γ8 being selectively enriched in the hippocampus.
Ingo’s group used electron cryo-microscopy to determine the structure of the GluA1/2 AMPA receptor in complex with TARP γ8 in a closed state. As well as showing the previously unknown arrangement of the subunits within this complex, high resolution visualisation of the pore provided a clearer understanding of the gating mechanism by which the channel allows sodium ions to flow through, but not calcium ions. The researchers also observed an extracellular loop of TARP γ8 that interacts with the part of GluA2 that binds to glutamate to modulate gating and found that disrupting this contact affected the function of the complex. Interestingly, the team were also able to see cell membrane lipids associated with the protein structure in the transmembrane domain, apparently stabilised by TARP γ8.
This work represents an important step forward in our understanding of the molecular biology behind memory formation and learning. In addition, recently developed epilepsy drugs are targeted against AMPA receptor/ TARP γ8 complexes, and this high resolution structure allowed Ingo’s group to visualise the drug binding site at a high level of detail. A detailed knowledge of the structure of the binding site could enable design of other new anti-epilepsy drugs that block AMPA function.
The work was funded by the MRC and Heptares Therapeutics.
Further references
Architecture of the heteromeric GluA1/2 AMPA receptor in complex with the auxiliary subunit TARP γ8. Herguedas, B., Watson, JF., Ho, H., Cais, O., Garcia-Nafria, J., Greger, IH. Science aav9011.
Ingo’s group page