Chris Oubridge, Senior Scientist in Kiyoshi Nagai’s group at the LMB for over 30 years, who made essential contributions to deciphering of the full reaction mechanism of pre-mRNA splicing, died on Tuesday 25th August 2020.
Chris began his scientific career studying microbiology at the University of Bristol, receiving his BSc in 1988 before joining Kiyoshi’s new group in the Structural Studies Division of the LMB. This was the start of a collaboration whose central and enduring theme would be the structural analysis of the spliceosome: the large RNA-protein complex that catalyses the excision of introns from messenger RNA precursors (pre-mRNAs) in eukaryotes. In the 1990s, structural analysis of the spliceosome was in its infancy, but Chris, Kiyoshi and their collaborators made an early breakthrough by determining the crystal structure of the RNA-binding domain of the U1 snRNP A protein, a key component of one of the RNA-protein complexes (so-called U1, U2, U4. U5 and U6 snRNPs) that are the major components of the spliceosome. This important advance was soon followed by the structure of the U1 A protein RNA-binding domain in a complex with its cognate RNA hairpin from the U1 snRNA, revealing crucial new insights into the molecular basis of sequence-specific RNA recognition by this family of RNA-binding proteins.
Running in parallel with these pioneering studies of the spliceosome, Chris also made important contributions to Kiyoshi’s other main project: structural analysis of the Signal Recognition Particle (SRP), the RNA-protein complex involved in translocation of secretory proteins across membranes. This work showed how SRP is assembled and how it recognises the signal peptide crucial for trans-membrane protein trafficking.
One of the pivotal advances made more recently by Chris, Kiyoshi and their collaborators involved reconstituting the human U1 snRNP from recombinant components and determining the crystal structure of the assembled RNA-protein complex. The structure revealed important clues about assembly of this snRNP complex and its biological role in recognition of intron 5’ splice sites. Another important advance came from solving the crystal structure of the crucial U5 snRNP protein Prp8, one of the most conserved spliceosomal proteins: the structure showed how Prp8 cradles the RNA-based active site of the spliceosome within a conserved electropositive cavity, and underscored intriguing evolutionary relationships between the spliceosome and distant relatives from the ancient RNA world.
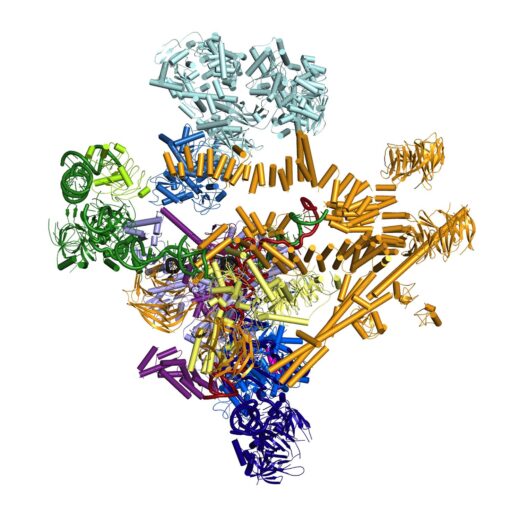
Just as crystallographic approaches seemed to be approaching a limit for structural analysis of the spliceosome, major advances in cryo-EM methods over the last few years, some of them pioneered at LMB, have opened up new opportunities for the structural analysis of complex biological assemblies, and Chris was heavily involved in many of the spliceosome projects that Kiyoshi’s group have recently pursued. These efforts have allowed the group to determine the cryo-EM structures of many of the key states of the spliceosome, revealing in great detail how the spliceosome is assembled, activated and remodelled to carry out catalysis of pre-mRNA splicing reactions.
Thirty-two years after starting his research career in Kiyoshi’s group at the LMB, Chris’ achievements are mapped out by a trail of landmark papers showing how some of the cell’s essential molecular machines do their work. He was a hugely talented experimental scientist and a thoroughly congenial lab-mate. Together with all this, Chris was an enthusiastic and perennial contributor to one of the great LMB traditions: the LMB Christmas party skit. His impish sense of humour and a keen eye for the (rare) absurdities of LMB life gave him just the right skills for excelling in this annual ritual of gently poking fun at his colleagues, whatever their station in LMB society. Chris was a treasured colleague and a great friend to many in the LMB community. He will be greatly missed.
Jan Löwe, Director of the LMB, commented: “Chris’ dedication, perseverance and talent as a staff biochemist and structural biologist enabled the astounding mechanisms of pre-mRNA splicing to be revealed by Kiyoshi Nagai’s group. His passing, as that of Kiyoshi only a few months ago, brings their conquest to a painful and premature end, but it may be some consolation that both saw the bulk of their discoveries completed.”