Apoptosis is a highly controlled form of cell death important for cell turnover during life, in embryonic development, including separation of fingers and toes, and as a cellular response against cancer. Although mitochondria are more widely known for their role as the energy-generating “powerhouses” of the cell, they also have an important role in initiating apoptosis: rupture of the mitochondria releases factors that contribute to an accelerating cascade towards cell death. This has been known for some time, but how such large holes are created in the outer mitochondrial membrane was not understood. Using cutting-edge correlative microscopy methods, Wanda Kukulski’s group in the LMB’s Cell Biology Division, in collaboration with Richard Youle’s group at the National Institutes of Health in the US, have now shed light on the process by which large mitochondrial ruptures form in initiation of apoptosis.
Mitochondria are made up of two membranes: an inner and an outer membrane. The inner membrane has many folds known as cristae. These folds allow the inner membrane to have a much greater surface area within the mitochondria, which is important in energy generation. The compartment between the two membranes, including the space within the cristae folds, contains components that drive apoptosis. The protein Bax plays a key role in rupturing mitochondria by aggregating in clusters on the outer membrane, which then leads to the formation of large holes in that membrane, allowing pro-apoptotic factors to leak out of the mitochondria and start cell death. However, it was unclear how such large holes were formed by these Bax clusters.
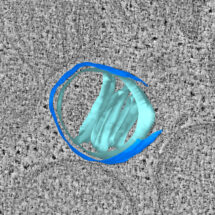
Video showing a 3D model of a mitochondrion during apoptosis, reconstructed from an electron cryo-tomogram. Two ruptures can be seen in the outer membrane (dark blue). Cristae folding of the inner membrane can be seen in light blue. Bax clusters are shown in red.
Using correlative microscopy, Nick Ader, a researcher from Wanda’s group, was able to visualise the cellular structures in mitochondria undergoing apoptosis in unprecedented detail. At its simplest, correlative microscopy involves performing fluorescence microscopy and electron tomography on the exact same sample, so that the data can be combined to allow very precise localisation of proteins such as Bax. A more novel correlative approach that Nick used for this study involved first rapidly freezing the cells, in a similar way to sample preparation in electron cryo-microscopy. Fluorescence microscopy was then used to identify which specific cell within the sample he wanted to investigate, and then a focused ion beam was used to produce a slice through that cell thin enough for electron cryo-tomography. This method is technically demanding, but results in the best resolution of cellular structure that is currently possible.
Observations made using these methods suggested a model on how such large holes can form in the mitochondrial membrane: on the one hand, Bax clusters create ruptures by removing components from the outer membrane, and on the other hand, the inner membrane unfolds where ruptures are present. As these events progress, the inner membrane unfolds further putting pressure on the outer membrane and increasing its propensity to rip open further. There are already cancer treatments that target the apoptotic pathway, of which Bax is a component, and understanding in detail the mechanism of apoptosis could help identify further therapeutic strategies that induce cell death in cancer cells.
This work was funded by the MRC, the NIH National Institute of Neurological Disorders and Stroke Intramural Research Program, the NIH-Oxford-Cambridge Scholars Program, and a Marshall Scholarship.
Further references:
Molecular and topological reorganizations in mitochondrial architecture interplay during Bax-mediated steps of apoptosis. Ader, NR., Hoffmann, PC., Ganeva, I., Borgeaud, AC., Wang, C., Youle, RJ., Kukulski, W. eLife Feb 4;8. pii: e40712.
Wanda Kukulski’s group page
Richard Youle’s group page