The bacterial SMC complex MukBEF uses a defined pathway to entrap DNA in advance of loop extrusion, which can be inhibited by bacteriophage protein gp5.9
Structural maintenance of chromosomes (SMC) complexes are large, ring-like molecular machines which organise chromosomes and aid diverse DNA transactions in all living organisms. They entrap DNA and extrude large DNA loops, but how exactly this occurs has remained unclear. Jan Löwe’s group, in the LMB’s Structural Studies Division, in collaboration with Mark Dillingham’s group at the University of Bristol, have set out to answer this.
Led by postdoc Frank Bürmann, now a Group Leader at the University of Oxford’s Department of Biochemistry, the groups studied bacterial MukBEF, found in E. coli and other related bacteria. They identified a comprehensive, directional loading mechanism, powered by adenosine triphosphate (ATP) hydrolysis, which MukBEF uses to capture and ingest DNA, and unexpectedly discovered how this process can be inhibited by the bacteriophage protein gp5.9.
To better examine the loading reaction of MukBEF, the team purified the complex and reconstituted the reaction in vitro, giving them a basis to conduct further investigations using biochemical and structural methods. In the absence of ATP, DNA entrapment was not observed and was abolished entirely in the case of a mutant form of the MukBEF complex which was ATP-hydrolysis deficient. This affirms that the reconstituted DNA loading reaction is driven by ATP hydrolysis, replicating the conditions observed in vivo.
The group conducted further tests to monitor the response of MukBEF to DNA with varying geometry. This revealed that MukBEF’s loading function is influenced by the topology of DNA, with a preference for relaxed DNA as opposed to tightly coiled or strained DNA.
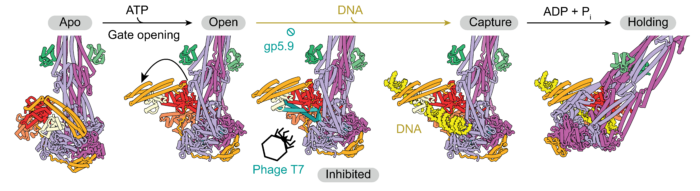
To more closely analyse MukBEF’s DNA transactions, the group used electron cryo-microscopy (cryo-EM) to visualise the processes. Structures of the complex without DNA bound, occurring early in the capturing process, revealed significant, structural alterations which prime for DNA receival. Specifically, upon ATP binding at the head, one of the subunits swings out away from the body of the complex by roughly 90 degrees, opening a wide gate at the neck region. This change only occurs when the heads of MukBEF are DNA-free, suggesting the complex senses it is ready to receive DNA. After opening the neck gate, DNA is then captured and bound at the periphery of the complex, but not yet fully entrapped.
Entrapment is instigated by ATP hydrolysis, which triggers the open neck gate to return to its ‘closed’ position through a large re-arrangement. The groups name this process the ‘stand-off and rotate’ model of DNA entrapment. This long-sought mechanism also explains why relaxed DNA is preferred, as it makes the double helix more accessible during rotation, which is therefore more efficient than tightly coiled DNA. This loading process readies the entrapped DNA for loop extrusion through additional rounds of ATP binding and hydrolysis. The model explains neatly why DNA entrapment stops ring opening in this case as it would lead to DNA release.
Finally, whilst investigating the molecular motivations of MukBEF’s DNA loading process, the groups serendipitously discovered a novel inhibitory factor. They found that a protein from a bacteriophage (a virus which infects bacteria) named gp5.9, interferes with MukBEF’s ability to load DNA. To understand this further, the team used cryo-EM to visualise the structure of MukBEF when bound to gp5.9, finding it binds to the MukE subunit. With the addition of gp5.9, the groups observed a strong inhibition of DNA ingestion. This is significant, as it reveals that viruses can directly interfere with the host’s chromosome structuring machinery.
This research has illuminated how MukBEF utilises a dedicated mechanism to open its neck gate to initiate the first stages of DNA capture. This starts a pathway of DNA loading, beginning at the neck gate and finishing with a ‘stand-off and rotate’ model to entrap the DNA before loop extrusion, which in its entirety only requires a single round of ATP hydrolysis. This represents an advancement in our understanding of DNA organisation in bacteria and may open doors for future clinical development to target bacterial growth.
The discovery of a dedicated DNA loading mechanism of an SMC complex also raises the possibility that other SMC complexes such as human cohesin and condensin entrap DNA in similar or analogous ways since their overall ring-like architectures are conserved.
This work was funded by UKRI MRC, EMBO, the Wellcome Trust, and UKRI BBSRC.
Since conducting this research, Frank has launched his own research group at the Department of Biochemistry and the University of Oxford where he continues to investigate bacterial genome remodelling machines.
Further references
Mechanism of DNA capture by the MukBEF SMC complex and its inhibition by a viral DNA mimic. Bürmann, F., Clifton, B., Koekemoer, S., Wilkinson, O.J., Kimanius, D., Dillingham, M.S., Löwe, J. Cell
Jan’s group page
Professor Mark Dillingham – University of Bristol
Frank Bürmann – University of Oxford
Previous Insight on Research articles
Atomic structure of chromosomal complex responsible for organising DNA determined
First complete atomic model of condensin lays foundation for understanding chromosome compaction