Manu Hegde’s group identifies a new complex that helps membrane proteins assemble correctly
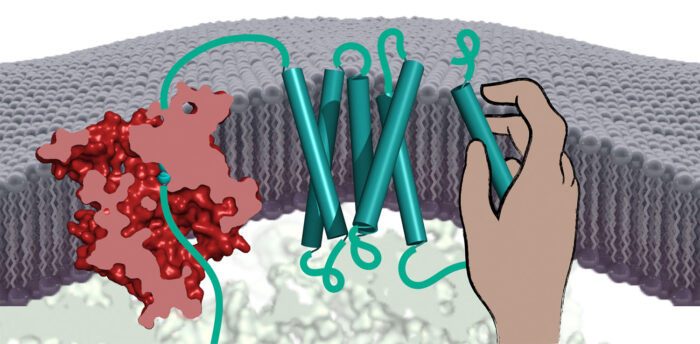
All cells rely on proteins embedded within their surrounding membrane to take up nutrients, communicate with neighbouring cells, expel waste, and respond to external stimuli. Most membrane proteins are folded into complex shapes that pass back and forth across the membrane many times. Patrick Chitwood and Manu Hegde, in the LMB’s Cell Biology Division, have discovered a new type of chaperone that helps membrane proteins assemble correctly.
To be stably embedded within membranes, membrane proteins contain hydrophobic stretches of amino acids that are not soluble in the cytoplasm of the cell and need to be inserted into a membrane. For this reason, nearly all membrane proteins are synthesised and assembled at the endoplasmic reticulum (ER), an interconnected membranous network that extends throughout the entire volume of a cell. The next challenge is to ensure that all membrane-spanning sections are correctly oriented across the membrane and assembled relative to each other. How this complicated assembly process works for all but the simplest membrane proteins has been unclear.
Previous work from Patrick, a PhD student in Manu’s lab, helped understand how the first membrane-spanning segment gets inserted into the membrane in the correct orientation. To investigate later steps in the insertion and assembly process, Patrick has now produced a series of incompletely assembled membrane protein intermediates in a test tube using ER membranes isolated from cells. Using chemical crosslinkers to trap any factors that might be involved in assembly led Patrick to a previously uncharacterised factor composed of two widely conserved proteins named Asterix and CCDC47. He named this factor the PAT complex for “protein associated with the translocon.”
Deletion of the PAT complex from human cells led to reduced successful production of many multi-spanning membrane proteins, suggesting a role for the PAT complex in their assembly. Returning to the test tube system, Patrick could show that the PAT complex binds to membrane-spanning segments of partially produced membrane proteins and lets go when the protein assembles into a stable structure.
In particular, the PAT complex was seen to bind to segments containing amino acids commonly found at the interface with other membrane-spanning segments. This suggests that the PAT complex’s job is to protect these vulnerable surfaces during the assembly process. Thus, the PAT complex seems to be a general intra-membrane chaperone that helps during the assembly of numerous membrane proteins.
Failure to correctly assemble particular membrane proteins underlies numerous human diseases such as cystic fibrosis, diabetes insipidus, retinitis pigmentosa and others. This probably explains why cells that lack the PAT complex show signs of ER stress and mice that lack the CCDC47 subunit of the PAT complex are embryonic lethal. Mutations in CCDC47 cause a severe developmental disease in humans. A deeper understanding of how membrane protein assembly occurs may eventually provide insights that help alleviate the disease consequences of impaired membrane proteins. From a practical standpoint, the PAT complex might be used to boost membrane protein production for functional, structural, or therapeutic purposes.
The work was funded by UKRI MRC.
Further references
An intermembrane chaperone complex facilitates membrane protein biogenesis. Chitwood, PJ., Hegde, RS. Nature (Epub ahead of print)
Manu’s group page