 Dynactin is a protein complex that activates the dynein motor protein, enabling intracellular transport. It is extremely flexible and has proved very difficult to study by conventional crystallography methods. Now for the first time, research carried out by Andrew Carter and his group in the LMB’s Structural Studies Division, has revealed the structure of this large dynactin complex, using electron cryo-microscopy (cryo-EM).
Dynactin is a protein complex that activates the dynein motor protein, enabling intracellular transport. It is extremely flexible and has proved very difficult to study by conventional crystallography methods. Now for the first time, research carried out by Andrew Carter and his group in the LMB’s Structural Studies Division, has revealed the structure of this large dynactin complex, using electron cryo-microscopy (cryo-EM).
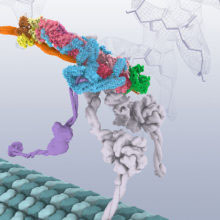
All cells contain an elaborate transport system to move important cargos to where they are needed. This transport system includes the dynein motor protein that moves along microtubules, the dynactin complex that allows the motor to walk for long distances, and an adaptor protein BICD2 that binds the cargo and glues these complexes together. This system is essential for the health of the cell, and mutations in dynein and dynactin can lead to neurodegenerative diseases.
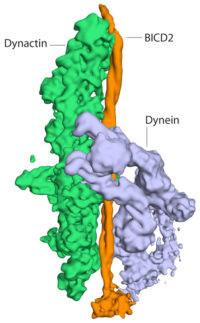 The dynactin complex is made up of 23 separate subunits and is difficult to produce. Linas Urnavicus and Kai Zhang purified dynactin from pig brains and worked out how to find different orientations of the flat dynactin samples in the electron microscope. They then used the Relion computer software, written at the LMB, to combine different views of the dynactin particles generating a 3 dimensional image. More information was provided by Aristides Diamant, with his crystallographic structure of part of the dynein motor, which helped the group to interpret the cryo-EM maps. Using the latest cryo-EM techniques, the group determined a high resolution structure of dynactin and a lower resolution structure of dynactin bound to dynein and BICD2.
The dynactin complex is made up of 23 separate subunits and is difficult to produce. Linas Urnavicus and Kai Zhang purified dynactin from pig brains and worked out how to find different orientations of the flat dynactin samples in the electron microscope. They then used the Relion computer software, written at the LMB, to combine different views of the dynactin particles generating a 3 dimensional image. More information was provided by Aristides Diamant, with his crystallographic structure of part of the dynein motor, which helped the group to interpret the cryo-EM maps. Using the latest cryo-EM techniques, the group determined a high resolution structure of dynactin and a lower resolution structure of dynactin bound to dynein and BICD2.
The structures show the BICD2 adaptor sandwiched between the other two complexes. Dynactin lies alongside it, and the dynein motor wraps around the outside of them both, opening up spaces between some of the dynein subunits as it curls around. This opening up of dynein suggests how the presence of dynactin could activate the motor. This work shows the power of cryo-EM to reveal structures that would have been almost impossible to solve only a few years ago, and highlights the importance of the rapid recent advances in cryo-EM.
Some viruses such as herpes and rabies manage to infect cells by hijacking the motor complex to get inside the cell and this structural information could help scientists design antiviral drugs. These structures may also allow us to understand the effects on the dynein motor of certain mutations that cause patients to get neurodegenerative diseases such as Motor Neuron Disease.
This work was funded by the MRC and the Wellcome Trust.