Dyneins are a family of motor proteins that run along the microtubule tracks that make up the cytoskeleton. They drive beating of cilia/flagellar and transport of cargos, contributing to processes such as clearing mucus, allowing sperm to swim, positioning organelles and clearing up misfolded proteins. All members of the family move along microtubules in the same direction, but it was not known why this is the case. A collaboration between Andrew Carter’s group in the LMB’s Structural Studies Division and researchers at University of California, Berkeley and Istanbul Technical University has used protein engineering, guided by molecular dynamics and electron cryo-microscopy (cryo-EM), to uncover how dynein’s structure determines directionality.
The two ends of a microtubule are known as the plus and minus ends. In a typical cell, the minus ends of microtubules are positioned at microtubule organising centres near the nucleus at the centre of the cell and the plus ends are near the cell edge. Dyneins all move towards the minus end of microtubules. Why this is the case was a question first discussed by Andrew and his collaborator Ahmet Yildiz in 2005 while they were both post-doctoral researchers at University of California, San Francisco.
Movement of the dynein motor is powered by chemical energy from ATP in a cycle that involves dynein binding and releasing from the microtubule in a process described as “walking”. Ten years after their first conversations about dynein’s directionality, better structures had shown how dynein binds to the microtubule through a long coiled stalk that is angled towards the direction of movement. This allowed Andrew and collaborators to predict that the angle of this interaction might be the reason for dynein’s movement. Over a meal at the 2016 Biophysics conference in Los Angeles, they set about planning how to produce variants of dynein with an altered stalk angle.
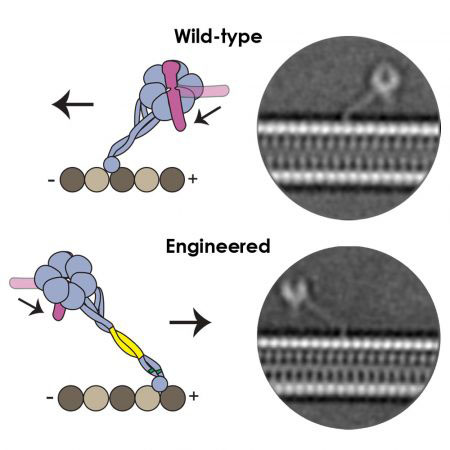
Guided by molecular dynamics Sinan Can from Ahmet’s group at University of California, Berkeley, engineered variants of dynein. Sam Lacey in Andrew’s group used cryo-EM to show that this reversed the angle and length of the stalk as predicted. Remarkably this change was sufficient to change the direction of movement of dynein, so that it now moved towards the plus end of the microtubule. Importantly, the length of the stalk and the angle at which it allows dynein to bind to microtubules is identical across a wide range of species, which provides an explanation for the fact that all dyneins move in the same direction.
There is a quote from the physicist Richard Feynman: “What I cannot create, I do not understand”. Applying this idea, Andrew’s group and their collaborators have demonstrated their understanding of dynein’s movement by designing variants that move differently. This was made possible by advances in protein engineering, molecular dynamics, and cryo-EM. Their innovative combined approach using all of these advances allowed the researchers to tackle questions that could not previously be answered and contributes to important advances in our understanding of the basic biology of many cellular processes, from clearance of mucus to collection of misfolded proteins.
This work was funded by the MRC, NIH, NSF, Wellcome Trust, and TUBITAK.
Further references:
Dynein’s directionality is controlled by the angle and length of its stalk. Can, S., Lacey, S., Gur, M., Carter, AP., Yildiz, A. Nature 566: 407-410
Andrew Carter’s group page
Ahmet Yildiz’s group page
Mert Gür’s group page