Understanding minute structural differences in GPCR (G protein-coupled receptor) complexes could lead to the design of more efficacious drugs that have fewer side effects
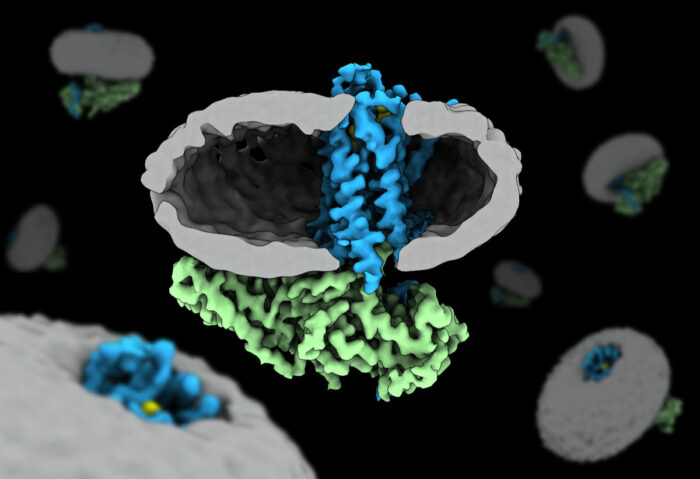
Yang Lee, Tony Warne and Rony Nehmé from Chris Tate’s group in the Structural Studies Division, and others, have solved the first high-resolution structure of arrestin coupled to a non-sensory GPCR. The β1-adrenoceptor (β1AR) is a GPCR activated by the hormone noradrenaline.
What are GPCRs?
G protein-coupled receptors (GPCRs) are integral membrane proteins that transduce chemical signals from the extracellular matrix into the cell. There are ~800 GPCRs encoded by the human genome and they respond to a diverse array of stimuli such as hormones, neurotransmitters, ions, photons and odorants. They play an important role in physiology and disease, and represent attractive drug targets.
Ligands that activate GPCRs may push signalling preferentially through either G proteins or arrestins in a phenomenon known as biased agonism. These different couplings are important in drug development, as the therapeutic effect may arise from only one signalling cascade, while the other pathway may mediate undesirable side effects. Unfortunately, the molecular basis for this phenomenon is poorly understood. However, there is good evidence that arrestin-biased ligands stabilise different subsets of receptor conformations compared to G protein-biased ligands.
GPCRs trigger critical intracellular signalling cascades through coupling with G proteins or arrestins – a process that is called ‘biased agonism’. A receptor can activate more than one signalling cascade and the one that is activated is determined by what activates it (in this case, either the G protein or the arrestin). Whichever triggers the receptor is an agonist and it biases the signalling in a particular direction. And certain ligands/drugs can bias this critical signalling towards either G proteins or arrestins.
GPCRs bind to various proteins (and form complexes) and it is quite difficult to catch them in these conformations to discern the differences among these complexes.
A large number of GPCR-G protein complexes have been determined by cryo-EM in recent years. However, structure determination of a GPCR-arrestin complex presents a greater challenge due to its smaller size, greater flexibility and specific requirements for complex formation.
Published in Nature, the key advance of the work is the comparison of the β1-adrenoceptor with the same ligand, coupled to either arrestin or a G protein mimetic at high enough resolution to observe subtle changes in the receptor. These minute differences are critical.
The coupling of arrestins to GPCRs is dependent on receptor phosphorylation. Starting with a thermostabilised form of the β1-adrenoceptor, the scientists engineered the end of the receptor to allow attachment of a synthetic phosphorylated peptide. This allowed them to produce a sample that was both highly and homogeneously phosphorylated.
The coupling of arrestin to the phosphorylated receptor proved inefficient when performed in detergent. To overcome this, they replaced the detergent that the receptor was purified in with a nanodisc – this is a discoidal lipid bilayer stabilised at its edges by a protein scaffold. Arrestins are known to interact with elements of the lipid bilayer when coupled to GPCRs. The nanodisc environment provides many of the essential factors.
To further stabilise the receptor-arrestin complex, they engineered arrestin with a mutation known to predispose it to its coupled state as well as introduced an antibody binder that recognises and locks it in said state. Having optimised the preparation biochemically, the sample was frozen in vitreous ice on cryo-EM grids for imaging on the Titan Krios.
With the help of electron microscopy and high-performance computing resources and expertise at the LMB, the researchers processed more than 18,000 images collected on the microscopes at the LMB and Diamond Light Source-eBIC in order to solve the structure.
As a proof of concept, this would be highly significant as many drug companies are trying to develop biased drugs to reduce side effects of current medications. A classic example of this is in pain therapy, where long-term use of opiates leads to chronic constipation, tolerance and addiction.
The work was funded by UKRI MRC, the Wellcome Trust, European Research Council, Department of Biotechnology, Indian Institute of Technology, Kanpur, India, Heptares Therapeutics, Council of Scientific and Industrial Research, India, BBSRC and the European Synchrotron Radiation Facility.
Further references:
Molecular basis of β-arrestin coupling to formoterol-bound β1-adrenoceptor. Lee, Y., Warne, T., Nehmé, R., Pandey, S., Dwivedi-Agnihotri, H., Chaturvedi, M., Edwards, PC., García-Nafría, J., Leslie, AGW., Shukla, AK., Tate, CG. Nature (Epub ahead of print)
Chris’ group page