 LMB scientists, Chris Tate and Yoko Shibata, have collaborated with researchers from the National Institutes of Health (NIH), USA, to provide the first detailed description of how a neuropeptide hormone, neurotensin, interacts with its receptor.
LMB scientists, Chris Tate and Yoko Shibata, have collaborated with researchers from the National Institutes of Health (NIH), USA, to provide the first detailed description of how a neuropeptide hormone, neurotensin, interacts with its receptor.
Neurotensin modulates nerve cell activity in the brain. When bound to its receptor it commences a series of reactions in nerve cells, and is involved in temperature regulation, pain and digestive processes. It is thought to be involved in neurological diseases, such as Parkinson’s disease and schizophrenia, as well as cancer and obesity.
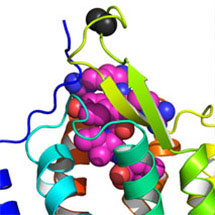
The research, lead by Reinhard Grisshammer’s laboratory at the National Institute of Neurological Disorders and Stroke, Maryland, USA, suggests that neuropeptide hormones use a novel binding mechanism to activate a class of receptors called G-protein coupled receptors (GPCRs). GPCRs are a large group of membrane proteins that are activated by a variety of molecules. The work, published this week in Nature, shows that neurotensin binds to the outer part of its GPCR, close to the receptor surface.
These results were obtained by using the latest X-ray crystallography techniques. Producing good quality, stable, neuropeptide-bound GPCR crystals is difficult. Chris Tate’s lab created a stable version of the neurotensin receptor that tightly binds neurotensin, by using recombinant gene technology.
This research has provided the first insight into the binding mode of a neuropeptide to a GPCR, and in future could help in the design of better drugs to treat neurological disorders, cancer and obesity.
This work was supported by the National Institutes of Health; Pfizer Global Research and Development; Medical Research Council; and the Protein Production Facility of the New York Consortium on Membrane Protein Structure, New York City.
Further references:
Article in Nature
Chris Tate’s Group Page
Nature News & Views Article