LMB researchers provide the first molecular framework that will help us understand and tune thyroid hormone production in health and disease.
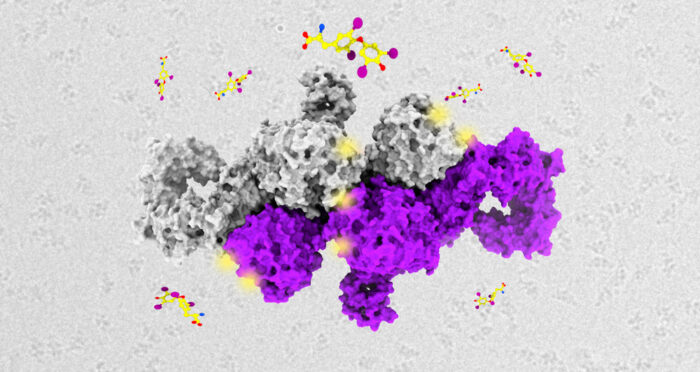
Thyroglobulin, the protein precursor to the thyroid hormones T3 and T4, is the only molecule in the human body that is modified by iodine, and the modification directly leads to the formation of the thyroid hormones in the thyroid gland. But the exact process has been sparsely understood. Now, a team of scientists including Jan Löwe, director of the LMB, Francesca Coscia, a researcher in Jan’s group in the LMB’s Structural Studies Division, and Veronica Chang, a member of Radu Aricescu’s group, have determined the first atomic structure of thyroglobulin (TG) and unraveled the molecular mechanism of thyroid hormone formation.
The TG atomic structure was imaged by single particle cryo-EM. In collaboration with Ajda Taler-Verčič, Ludwig Sinn, Francis J. O’Reilly, Thierry Izoré, Miha Renko, Imre Berger, Juri Rappsilber and Dušan Turk, the LMB team combined the mapped model with other methods – mass spectrometry data, mutagenesis and in vitro hormone production – that allowed identification of the tyrosine amino acids involved in hormone synthesis. Thereafter, by transferring the TG reaction properties to a completely unrelated bacterial protein (MBP or maltose binding protein), the scientists were able to synthesise hormones with the same efficiency as in TG.
The discovery of the structure is important because it ultimately determines the exact hormone yield from one thyroglobulin molecule and also shows that this mechanism can be mimicked by other tyrosine-containing molecules. TG is the most abundant protein in the thyroid and is believed to regulate hormone production via regulation of its gene expression, supramolecular structure and interaction with other molecular partners.
Our bodies need iodine only because it is required to produce thyroid hormones from TG. And because of this unique chemical feature, T3 and T4 can act on metabolism and gene expression with very high specificity throughout the entire body. “It will be fascinating to understand when in evolution this function was firstly developed as it is so unique to vertebrates,” says Francesca.
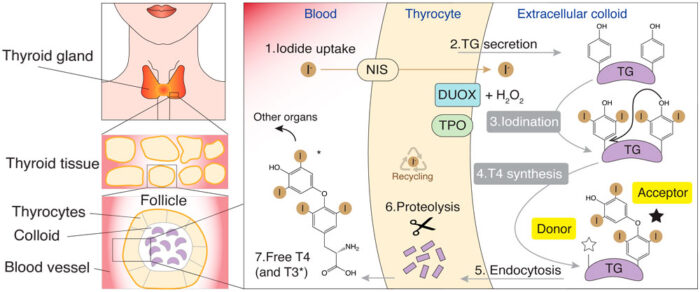
What does the protein do?
Thyroglobulin is a complex and large molecule, from which only seven molecules of the hormone are produced using a chemical reaction that could be carried out by less complex proteins. However, in the context of the iodine cycle in vertebrates, the thyroglobulin structure combines hormone production with iodination of amino acids (tyrosine residues) for iodine storage. Moreover, the complexity of the TG molecule might fulfil further important roles in endocytosis, regulation of thyroid hormone ratio (T3 and T4), TG proteolytic processing and in the trafficking to lysosomes. The atomic structure will enable further studies towards a deeper understanding of thyroglobulin within the thyroid, and its involvement in thyroid diseases.
Thyroid diseases affect 5% of the world’s population, particularly women and young children, with mental retardation, metabolic and cardiovascular problems. TG is also involved in the development of auto-immune diseases and is the main thyroid cancer marker in blood. In the worst cases the thyroid has to be surgically removed with considerable impact on well-being and life style. Understanding TG and thyroid hormones will help in the design of better diagnosis and treatments of thyroid disorders, and possible alternatives (better ways of making thyroid hormones) to suboptimal hormone or iodine prescription and/or thyroid surgical removal.
The work was funded by the MRC, the Wellcome Trust and the Slovenian Research Agency.
Further references:
The structure of human thyroglobulin. Coscia, F., Taler-Verčič, A., Chang, VT., Sinn, L., O’Reilly, FJ., Izoré, T., Renko, M., Berger, I., Rappsilber, J., Turk, D., Löwe, J. Nature doi: 10.1038/s41586-020-1995-4
Jan’s Group Page