The high-resolution structure of Ste2 sheds light on how GPCR dimerisation can occur and provides the template for future understanding of other fungal GPCRs
G protein-coupled receptors (GPCRs) are membrane proteins that transduce signals from a wide range of stimuli. They are also the targets of approximately 34% of all FDA-approved drugs, due to their wide-ranging important functions in our cells. Design of new, improved drugs with fewer side-effects requires a fuller understanding of their structures. GPCRs in fungi could also be useful drug targets, but no structures of fungal GPCRs have previously been solved. Chris Tate’s group, in the LMB’s Structural Studies Division, has now determined the first structure of a fungal GPCR.
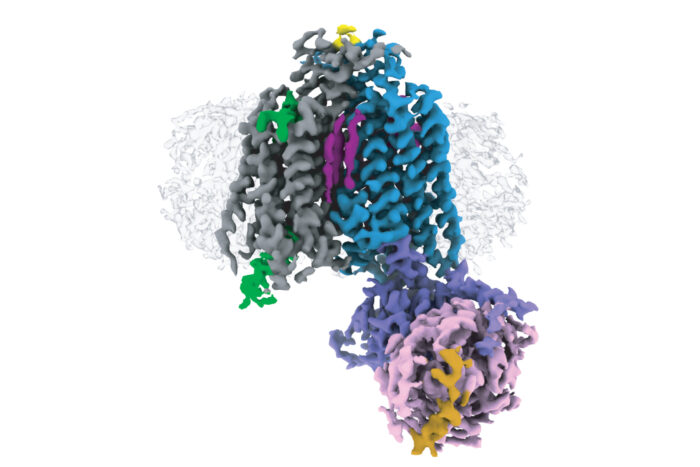
GPCRs can be divided into six classes, A – F, based on their evolutionary relationships to each other. Electron cryo-microscopy (cryo-EM) and X-ray crystallography have enabled determination of over 370 structures of vertebrate GPCRs in classes A, B, C, and F. Class D GPCRs are found exclusively in fungi where they regulate survival and reproduction. Vaithish Velazhahan, a PhD student in Chris’ group, set out to solve the structure of the class D GPCR Ste2, which is a pheromone receptor in Saccharomyces cerevisiae yeast.
After engineering a version of a yeast G protein that would capture Ste2 in an active state and developing methods to purify and then assemble the required components, Vaithish used cryo-EM to solve the structure of a Ste2 dimer in an active state and coupled to two G proteins. Molecular dynamics simulations performed in collaboration with Nagarajan Vaidehi’s group at the Beckman Research Institute of the City of Hope, USA, showed an unexpected mobility of one of the G proteins, which is now under further study.
As the first structure of a fungal GPCR, this structure provides a template for the understanding of other class D GPCRs and for the design of novel drugs targeting fungal GPCRs. In collaboration with David Gloriam’s group at the University of Copenhagen, Denmark, a unified nomenclature for Class D1 GPCRs was developed to facilitate future work.
This is also the first high-resolution structure of a GPCR dimer where the interface involves extensive interactions within the transmembrane region. High-resolution structures of GPCR dimers have only previously been determined for class C GPCRs, which exist as constitutive dimers due to interactions between large extracellular venus flytrap domains that are absent in other GPCR classes. Whether other classes form physiologically relevant dimers had been a controversial debate for many decades, with most scientists now accepting that dimers form through transient interactions between transmembrane domains. This structure sheds light on how such an interaction can take place.
The tools developed for this piece of work are expected to provide a foundation for structure determination and understanding of other fungal GPCRs, including those implicated in several major fungal diseases. As this structure shows that the ligand binding pocket and binding mode in Ste2 is considerably different from human GPCRs, it might be possible to design specific drugs that target fungal GPCRs involved in prevalent fungal diseases, such as candidiasis, or that can reduce crop spoilage caused by fungal pathogens.
The work was funded by UKRI MRC, Gates Cambridge Trust, ERC, Sosei Heptares, Lunbeckfonden, Novo Nordisk Fonden, Independent Research Fund Denmark, and NIH.
Further references
Structure of the class D GPCR Ste2 dimer coupled to two G proteins. Velazhahan, V., Ma, N., Pándy-Szekeres, G., Kooistra, AJ., Lee, Y., Gloriam, DE., Vaidehi, N., Tate, CG. Nature https://doi.org/10.1038/s41586-020-2994-1
Chris’ group page
Nagarajan Vaidehi’s group page
David Gloriam’s group page