Cryo-EM structures of α-synuclein filaments may benefit diagnosis and treatment of neurodegenerative synucleinopathies
The presence of abnormal filaments of the protein α-synuclein in brain cells defines a number of neurodegenerative diseases, including multiple system atrophy (MSA), Parkinson’s disease, Parkinson’s disease with dementia and dementia with Lewy bodies. However, the structures of the filaments formed by α-synuclein in the brains of patients with these diseases are not known. New research from the groups of Michel Goedert and Sjors Scheres has revealed the first structures of α-synuclein filaments from human brain.
MSA is a neurodegenerative disease with symptoms of parkinsonism, cerebellar ataxia, and autonomic failure. It is defined by regional nerve cell loss and the presence of abundant filamentous α-synuclein inclusions in non-neuronal cells in the brain; smaller numbers of inclusions are also seen in nerve cells. Until this study, the structures of these filaments from human brain were unknown.
Working with α-synuclein filament preparations that had been characterised and extracted by Masato Hasegawa’s group at the Tokyo Metropolitan Institute of Medical Science, Manuel Schweighauser and Yang Shi used cryo-EM to solve the structures of α-synuclein filaments from the brains of five patients who had died with MSA. Importantly these structures are different from those of recombinant α-synuclein filaments, showing the importance of working with human brain tissue.
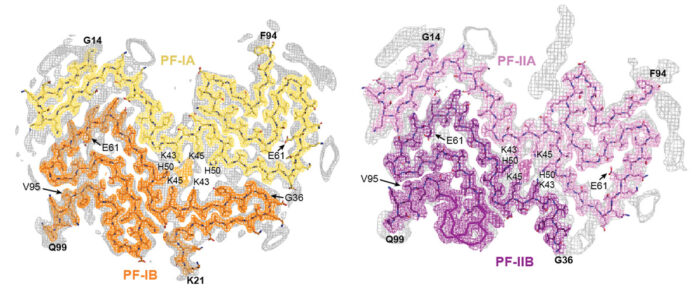
The team found that there are two types of α-synuclein filament and that each type is made of two different protofilaments, showing that the filaments are asymmetric. This means that in MSA filaments α-synuclein adopts four different protofilament folds, such that one pair of protofilaments makes up one type of filament and another pair makes up the second type of filament. This asymmetry contrasts with the structures of tau filaments previously obtained by Sjors’ and Michel’s groups in which only one protofilament fold was found in each disease investigated. Another difference with tau filaments was the much larger interface between the two protofilaments of α-synuclein.
Interestingly, both α-synuclein filament types contain a cavity between the two protofilaments in which non-protein molecules of unknown identity are present. Non-protein molecules were also previously seen within cavities inside tau filaments from the brains of patients with chronic traumatic encephalopathy and corticobasal degeneration. It is possible that the contents of these cavities play a role in filament formation. Work is underway to determine the identities of these non-protein molecules.
As well as investigating α-synuclein filaments from patients with MSA, the researchers also used two-dimensional analysis of α-synuclein filaments from three patients with dementia with Lewy bodies to show that the structures of these filaments are probably distinct from those of MSA. This suggests that different strains of α-synuclein filaments characterise specific synucleinopathies, as has been seen for tau filament structures in different tauopathies.
This work extends the team’s work on tau filaments and confirms that cryo-EM can be used to determine the structures of amyloid filaments from human brain, opening the way to determination of the structures of α-synuclein filaments from the brains of individuals with Parkinson’s disease. Imaging α-synuclein inclusions in the living brain is not currently possible, but these structures might enable development of PET imaging ligands to allow accurate diagnosis. It may also be possible to use these structures for the design of molecules that prevent, inhibit, or reverse formation of α-synuclein filaments for treatment of MSA.
The work was funded by UKRI MRC, Eli Lilly and Company, the European Union, the Japan Agency for Medical Research and Development, the US National Institutes of Health, and the Department of Pathology and Laboratory Medicine at Indiana University School of Medicine.
Further references
Structures of α-synuclein filaments from multiple system atrophy. Schweighauser, M., Shi, Y., Tarutani, A., Kametani, F., Murzin, AG., Ghetti, B., Matsubara, T., Tomita, T., Ando, T., Hasegawa, K., Murayama, S., Yoshida, M., Hasegawa, M., Scheres, SHW., Goedert, M. Nature (Epub ahead of print)
Michel’s group page
Sjors’ group page
Masato Hasegawa’s page
ALZFORUM: Behold the First Human α-Synuclein CryoEM Fibril Structure
Previous Insight on Research
A novel tau fold in the neurodegenerative disease corticobasal degeneration