Scientists in Leo James’ group in the LMB’s PNAC Division, in collaboration with Till Böcking’s group at the University of New South Wales, Australia and Adolfo Saiardi’s group at the MRC Laboratory for Molecular Cell Biology, have uncovered how the HIV virus stabilises its capsid by binding to an abundant cellular polyanion, IP6. IP6 increases HIV capsid stability from minutes to hours, allowing HIV to copy its RNA genome into DNA inside the capsid with the result that this newly synthesised DNA genome is protected from destruction by the cell.
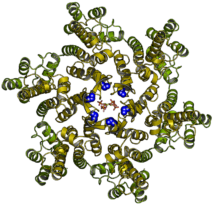
Viruses, like HIV, hide from our immune system. To do this, HIV uses a protein shell called a capsid, which surrounds its nucleic acid genome and protects it from detection and destruction. The capsid must be strong enough to survive for hours inside the cell’s cytosol whilst also being able to open quickly when the virus needs to release its genome. How this process, called ‘uncoating’, is achieved is one of the great unanswered questions in HIV biology. Previously, Leo’s group made the unexpected discovery that the HIV capsid is decorated with hundreds of pores. Each pore contains a ring of six positively charged residues – this should be strongly destabilizing yet the pore is one of the most highly conserved features across the group of viruses to which HIV belongs, suggesting that the pores must have some essential function. Donna Mallery from Leo’s group therefore set out to investigate why the HIV capsid contains these positively-charged pores and what they might be for.
Using a combination of cellular virology and biochemistry, Donna found that the HIV capsid pores bind an abundant cellular polyanion called IP6. Using X-ray crystallography, she revealed how the six negatively charged phosphates in IP6 match the six positively charged residues in the pore. By employing a new method that allows the fate of individual capsids to be visualized through time, Donna collaborated with Till Böcking to discover that IP6 increases capsid stability from just minutes to over ten hours. Importantly, they found that this allows HIV to copy its RNA genome into DNA inside the capsid, meaning the newly synthesized genome is protected from cellular nucleases. Finally, Donna teamed up with Adolfo Saiardi to devise a way to test whether IP6 is packaged into HIV virions when they are produced from infected cells. Together they discovered that each HIV virus packages itself with over 300 IP6 molecules.
Another type of virus, picornaviruses, solve the problem of capsid stability by using small molecules called pocket factors. These stabilize the capsid when bound and trigger uncoating when they dissociate. This new work by Leo’s group suggests that IP6 is an HIV pocket factor, used by the virus to assemble its capsid, maintain stability and provide a trigger for uncoating. Just as pocket factors have led to the development of anti-picornavirus drugs, the discovery of this missing piece of HIV biology may help guide new types of antiretroviral therapy.
Further references:
Paper in eLife
Leo’s group page
Till Böcking’s group page
Adolfo Saiardi’s group page