 Research carried out by Anne Bertolotti’s group in the LMB’s Neurobiology Division has identified a novel protein, named Adc17, that acts as an inducible chaperone to help cells make more proteasome when needed.
Research carried out by Anne Bertolotti’s group in the LMB’s Neurobiology Division has identified a novel protein, named Adc17, that acts as an inducible chaperone to help cells make more proteasome when needed.
Cells and organisms constantly need to adapt to maintain protein homeostasis under adverse stress conditions in order to avoid cell death. Cells have evolved numerous and sophisticated protein quality control systems to adapt to changes in their environment. However, sometimes these systems get overwhelmed, when the demand is too high. This is particularly the case during ageing and in some age-related neurodegenerative diseases which are characterised by accumulation of misfolded proteins that should have been degraded.
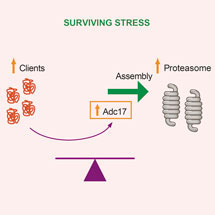
The proteasome is essential for the selective degradation of most cellular proteins. Overwhelming demands on the proteasome represents a stress and to survive such stress cells need to increase proteasome abundance. Using a genetic screen in yeast, Anne’s group found that increasing expression of Adc17 enabled cells to survive proteasome stresses. The abundance of Adc17 increases upon proteasome stress to assist an early step in proteasome assembly: the pairing of two of its subunits, Rpt6 and Rpt3. Adc17 is not only important to survive proteasome stress but also for cell fitness as it is crucial to maintain homeostatic levels of proteasomes to match demand.
Identifying pathways to survive protein quality control failure is important because it may help identify strategies to ameliorate the large number of pathologies associated with accumulation of unwanted proteins.
This work was supported by the Medical Research Council, the Motor Neurone Disease Association, the European Molecular Biology Organization and the Human Frontier Science Program.
Further References:
Paper in Molecular CellBaumann, Kim. Research Highlight – How the proteasome adapts to stress. Nature Reviews Molecular Cell Biology
Anne’s Group Page