Investigations into Cockayne Syndrome has revealed how formaldehyde triggers an anorexic DNA damage response leading to the release of appetite suppression hormone GDF15
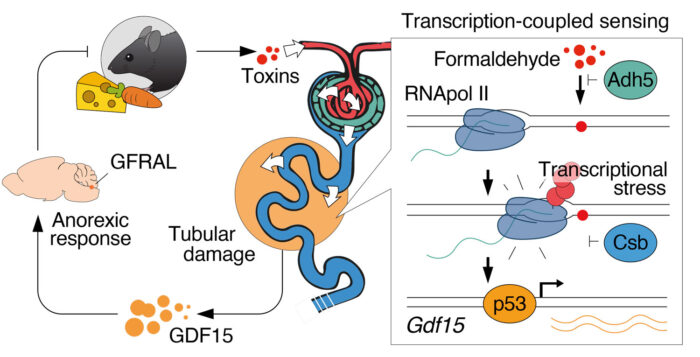
Cockayne Syndrome (CS) is an inherited, genetic disease characterised by severe weight loss, kidney dysfunction, neurological abnormalities, and a prematurely aged appearance. CS occurs due to mutations in the genes CSA and CSB which have long been identified as critical to repairing DNA during the transcription process of DNA to RNA. However, the source of DNA damage that requires repair by CSA and CSB has remained elusive. Now, KJ Patel (previously at the LMB, now at the MRC Weatherall Institute of Molecular Medicine at the University of Oxford) and his group have identified how naturally-occurring formaldehyde damages DNA in a manner requiring CSA and CSB. The study, which was largely conducted at the LMB, involved a multi-group collaboration including Gerry Crossan’s group in the LMB’s PNAC Division, Menna Clatworthy’s group from the University of Cambridge Molecular Immunity Unit which is based at the LMB and Juan Garaycoechea’s group at the Hubrecht Institute.
In looking to answer why a failure to fix DNA damage causes the severe symptoms of CS, the group studied genetically-engineered mice with a mutated CSB gene which display none of the features of CS. Further engineering to introduce formaldehyde – a molecule KJ’s group has previously shown is present within the body, partially from a dietary source – left the mice unable to clear the formaldehyde and also unable to repair damaged DNA. The mice developed symptoms of human CS, including kidney failure and severe weight loss.
Using advanced single cell sequencing to study the kidney of the animal model, the researchers identified the kidney proximal tubules as the critical cellular site where DNA damage occurs. This damage causes the cells to secrete growth differentiation factor 15 (GDF15), an anorexic factor which stimulates weight loss by driving a signal to the brain to trigger appetite suppression. As the dramatic weight loss in CS patients is similar to weight loss in patients undergoing chemotherapy, the group also treated mice models with the chemotherapeutic agent Cis-Platin. Interestingly, this also activated the kidney cells to release GDF15 into the blood stream. Importantly, when the group administered an antibody to GDF15 to block this signaling response, the mouse model was able to gain weight again.
The findings suggest that, upon the consumption of toxic food or treatment with chemotherapy, toxins such as formaldehyde enter the blood stream and are filtered by the kidney. The toxic formaldehyde then damages the DNA in kidney cells which prompts the secretion of GDF15 into the blood, thereby triggering an appetite suppression response in the brain. In CS patients, the failure to repair the damaged DNA in the kidney means the release of GDF15 is never stopped. This explains the relentless weight loss associated with this disease.
This paper is significant as its findings explain the physiological purpose of CSA and CSB; to repair DNA damage caused by formaldehyde. The authors have also outlined a mechanism by which people with CS fail to gain weight. The mechanism – the release of GDF15 from kidney cells with DNA damage – is also likely relevant in other contexts, including chemotherapeutic treatments. This therefore holds important clinical implications to treat weight loss in patients undergoing cancer chemotherapy and in children with CS, through administration of an antibody which neutralises GDF15.
More generally, the authors speculate that this food aversion response might have evolved in mammals to provide protection against the ingestion of toxic food sources (such as formaldehyde) so that no further toxins are ingested while DNA repair occurs.
This work was funded by UKRI MRC, Jeffrey Cheah Foundation, CRUK, Wellcome Trust, University of Cambridge, Hubrecht Institute, NIHR and the European Union Research and Innovation programme Horizon 2020.
Further references
Aldehyde-driven transcriptional stress triggers an anorexic DNA damage response. Mulderrig, L., Garaycoechea, JI., Tuong, ZK., Millington, CL., Dingler, FA., Ferdinand, JR., Gaul, L., Tadross, JA., Arends, MJ., O’Rahilly, S., Crossan, GP., Clatworthy, MR., Patel, KJ. Nature
KJ Patel’s group page
Juan Garaycoechea’s group page
Menna Clatworthy’s group page
Gerry Crossan’s group page
MRC Weatherall Institute of Molecular Medicine news release
Previous Insight on Research articles
Uncovering how alcohol-derived metabolites damage the genome of stem cells
Folate and formaldehyde: from vitamin to genotoxin to DNA building block
A fundamental protection mechanism against formalin in mammals is revealed