Cryo-EM reveals both neurodegenerative diseases possess identical Lewy fold structure
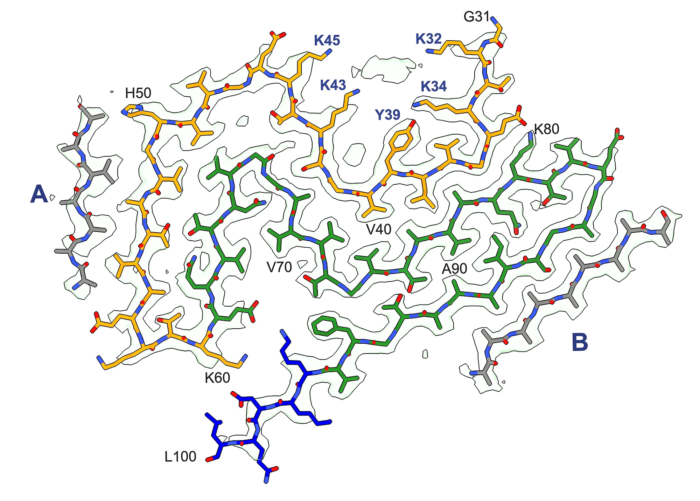
Building on a long-standing collaboration, the groups of Michel Goedert and Sjors Scheres, from the LMB’s Neurobiology and Structural Studies Divisions, have used electron cryo-microscopy (cryo-EM) to determine the structures of α-synuclein filaments from the brains of individuals with Parkinson’s disease and dementia with Lewy bodies. Their formation is believed to cause nerve cell degeneration and the symptoms of these diseases.
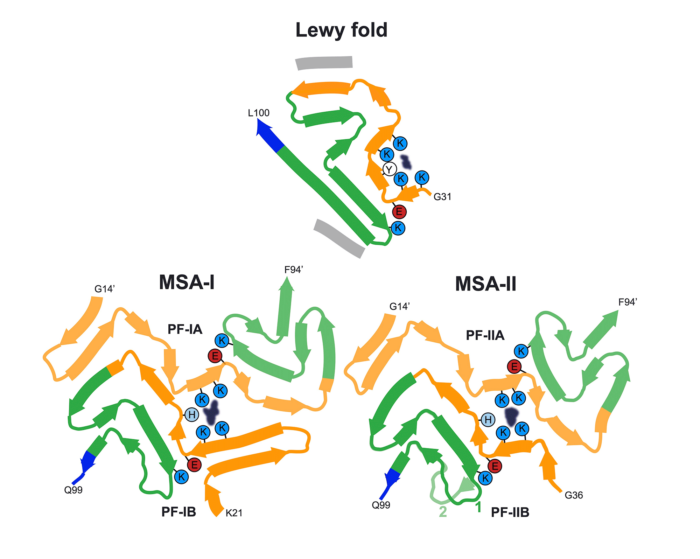
These findings are highly significant, as they report the previously unknown structure of α-synuclein filaments from the Lewy pathology of Parkinson’s disease – the second most common neurodegenerative disease and the most common movement disorder. Notably, the group also found that this filament structure is the same as that from the brains of individuals with dementia with Lewy bodies. They have named this α-synuclein structure the Lewy fold.
The finding of identical structures of α-synuclein filaments in both diseases shows that they are on a continuum. This is not true of some other neurodegenerative diseases characterised by the presence of abundant α-synuclein filaments. Thus, the group have previously determined the structures of filaments from multiple system atrophy (MSA), which were very different from the Lewy fold.
This new study establishes the existence of multiple molecular conformers of synucleinopathies. With this research, the group have now determined the folds of assembled α-synuclein from the three major synucleinopathies: Parkinson’s disease, dementia with Lewy bodies and MSA.
Knowledge of different α-synuclein filament structures will aid in the development of better diagnostics for Lewy body diseases and MSA. It may now be possible to use structure-based design of small molecules that can be developed as positron emission tomography ligands specific for Lewy and MSA folds. Such ligands are necessary for the presymptomatic detection of α-synuclein assemblies in human brain, which, together with mechanism-based therapies, is an essential prerequisite for the prevention of disease symptoms. The new work also opens the way to the development of methods by which to produce α-synuclein filaments with structures identical to those in human brains. In the long run, this could lead to the development of novel mechanism-based therapies for Parkinson’s disease and dementia with Lewy bodies.
Lead author Yang Yang was assisted by Yang Shi and Manuel Schweighauser (all LMB postdocs) in conducting the cryo-EM analysis. The team used advanced electron microscopes, made available by Abhay Kotecha at Thermo Fisher Scientific. Brain samples were provided by Tammaryn Lashley from UCL Queen Square Institute of Neurology, Masato Hasegawa from Tokyo Metropolitan Institute of Medical Science, and Bernardino Ghetti from Indiana University School of Medicine.
This work was funded by UKRI MRC, Alzheimer’s Research UK, the National Institute for Health Research Queen Square Biomedical Research Unit in Dementia, the Rita Lila Weston Institute for Neurological Studies, the Japan Agency for Science and Technology (CREST), the Japan Agency for Medical Research and Development (AMED), the US National Institutes of Health, and the Department of Pathology and Laboratory Medicine, Indiana University School of Medicine.
Further references
Structures of α-synuclein filaments from human brains with Lewy pathology. Yang, Y., Shi, Y., Schweighauser, M., Zhang, X., Kotecha, A., Murzin, AG., Garringer, HJ., Cullinane, PW., Saito, Y., Foroud, T., Warner, TT., Hasegawa, K., Vidal, R., Murayama, S., Revesz, T., Ghetti, B., Hasegawa, M., Lashley, T., Scheres, SHW.*, Goedert, M*. Nature *Joint supervision
Michel’s group page
Sjors’ group page
Tammaryn Lashley’s page
Masato Hasegawa’s page
Bernadino Ghetti’s page
Previous Insight on Research articles
First structures of α-synuclein filaments from human brain
Atomic structures of Aβ42 filaments from the brains of individuals with Alzheimer’s disease and other neurodegenerative conditions
TMEM106B filaments form in an age-dependent manner in human brains