 Target of Rapamycin (TOR) is a protein kinase that is essential in maintaining cellular homeostasis. In mammalian cells the enzyme occurs as two large protein complexes and one of these, mTORC1, controls growth of cells by integrating signals from growth factors and the nutritional state of cells. Many tumours in humans are associated with inappropriate regulation of this protein complex, and it has been demonstrated to be an important therapeutic target for cancer and autoimmune disorders. Work by Roger Williams’ group in the LMB’s PNAC Division on the structure of this complex is helping us to understand the function of this important regulatory complex.
Target of Rapamycin (TOR) is a protein kinase that is essential in maintaining cellular homeostasis. In mammalian cells the enzyme occurs as two large protein complexes and one of these, mTORC1, controls growth of cells by integrating signals from growth factors and the nutritional state of cells. Many tumours in humans are associated with inappropriate regulation of this protein complex, and it has been demonstrated to be an important therapeutic target for cancer and autoimmune disorders. Work by Roger Williams’ group in the LMB’s PNAC Division on the structure of this complex is helping us to understand the function of this important regulatory complex.
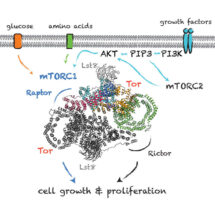
TOR is present in two complexes, mTORC1 and mTORC2, in which the TOR–Lst8 heterodimer forms a common sub-complex. Using the great advances in electron cryo-microscopy (cryo-EM), Domagoj Baretić, Alex Berndt and Yohei Ohashi in Roger’s group carried out the work that led to the structure of a complex of TOR bound to Lst8. The structure has provided insight into the complex three-dimensional topology of this enzyme and will act as a guide for future work on this growth-regulating pathway. Roger’s group will now be using the structure of this complex to understand how it is regulated and how diverse regulatory influences affect its function.
Due to the role of TOR in the cell, mTOR inhibitors could have important roles in suppressing transplant rejection and autoimmunity as well as treating obesity and cancer. Inhibitors of mTORC1 have become standards in treating some types of cancers, and are able to prolong lifespan in mice. Inhibitors that specifically control the regulation of mTORC1 in response to growth factors might minimise the toxicity of these inhibitors and this could be important for their application in life-span extension. Knowing more about the structure and function of TOR could facilitate design of better therapeutic agents.
The work was funded by the MRC.