Our genome contains DNA from ancestral retroviral infections. These stretches of DNA are not usually harmful unless the cell’s normal ability to regulate them is lost, then their expression can potentially lead to disease. Yorgo Modis’ group, in the University of Cambridge Molecular Immunity Unit at the LMB, have solved the structure of a master regulator of integrated retroviral DNA, KAP1, providing mechanistic understanding into the function of KAP1 in silencing retroviral insertions.
Many viruses, including HIV and other retroviruses, can integrate their DNA into our DNA after infecting our cells. As viruses have likely been evolving alongside their hosts since the origin of life on Earth, retroviral DNA inherited from ancestral infections accounts for approximately 10% of our genome. Interestingly, although we tend to only think of viruses as being pathogens, our long co-existence has led to some instances of virally derived DNA developing useful functions within our cells. For example, some genes involved in development of the placenta and certain DNA sequences that regulate the differentiation of stem cells into specialised cells are derived from viral genes. However, many virally-derived regions must be tightly regulated to restrict their proliferation and prevent toxic gene expression. KAP1 is the primary regulator of these retrovirus insertions.
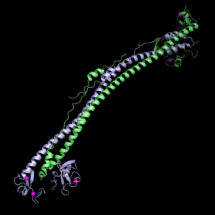
Guido Stoll, a PhD student in Yorgo’s group, used X-ray crystallography to determine the structure of the part of KAP1 that recognises integrated viral DNA at atomic-level detail. KAP1 regulates gene expression as an epigenetic repressor, which involves putting a chemical label on the DNA that makes it less likely to be expressed. Yorgo’s group were able to confirm which specific parts of the surface of KAP1 were functionally required for epigenetic silencing. This involved introducing mutations affecting particular parts of the surface through which KAP1 is recruited to viral DNA and measuring a loss of KAP1 silencing activity in a cell-culture assay. These findings will serve as the basis for expanding our mechanistic understanding of the epigenetic mechanisms used to silence genomic sequences of viral origin.
Due to their mutagenic nature, virus-derived DNA fragments that have been integrated into the human genome have the potential to cause disease (via retrotransposition events) if they escape repression. Retrotransposition events can disrupt protein coding sequences, and retrotransposon activity is associated with a range of diseases, including cancer, haemophilia, cystic fibrosis, a form of age-related macular degeneration known as geographic atrophy, and lupus. An improved understanding of how KAP1 functions in healthy individuals could suggest new strategies for treatment of these diseases. In the longer term, these structures could aid with the design of small molecule drugs that inhibit or modulate the expression of genes in retrovirally-derived sequences.
The work was funded by the Wellcome Trust, MRC and BBSRC.
Further references
Structure of KAP1 tripartite motif identifies molecular interfaces required for retroelement silencing. Stoll, GA., Oda, S-I., Chong, Z-S., Yu, M., McLaughlin, SH., Modis, Y. PNAS [Epub, DOI: 10.1073/pnas.1901318116 ]
Yorgo’s group page