High-throughput CRISPR screening has allowed research into what drives and regulates IL-13 production by TH2 cells during an allergic immune response
The body’s immune system is designed to recognise what is ‘self’ and thereby keep out danger of infection or disease from pathogens. Allergy and allergic asthma result from inappropriate immune reactions. According to the charity Allergy UK, allergic diseases affect 20% of the UK and world population. Although mild allergies such as hay fever can be treated with over-the-counter antihistamines, severe diseases require other treatment options which target more upstream pathways to the symptoms alone.
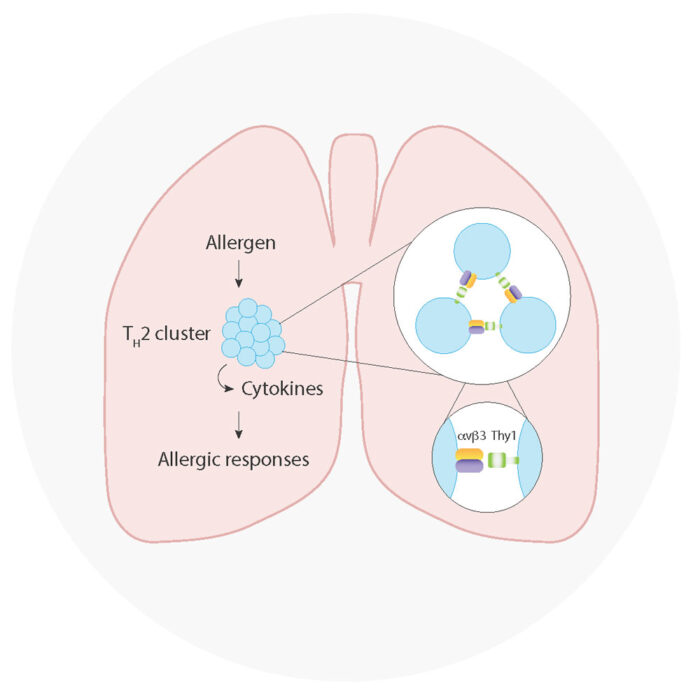
Researchers in Andrew McKenzie’s group in the LMB’s PNAC Division have now identified that a molecule called alpha V beta 3 (αvβ3) integrin on T helper 2 (TH2) cells mediates cellular clustering and cytokine production, driving allergic inflammation. It is known that TH2 cell production of interleukin-13 (IL-13) drives asthma and allergic diseases through unwanted inflammation, but the current understanding of how IL-13 production is regulated remains incomplete.
Aydan Szeto, PhD student and now postdoc in the McKenzie group, with Patrycja Kozik from the LMB’s PNAC Division, used high-throughput CRISPR screening to simultaneously assay all known genes in the mouse genome (~20,000) for their involvement in IL-13 production. The screens identified a protein module on TH2 cells consisting of a pair of cell surface proteins called αvβ3 integrin.
To verify and understand the involvement of αvβ3 integrin in the immune response, Aydan together with the wider McKenzie group, used in vivo mouse models of lung allergy to confirm that the integrins are important for allergic responses. Furthermore, mice lacking αvβ3 integrin, specifically in T cells, produced lower amounts of IL-13, curtailing allergic inflammation.
Further cell-based inhibitor assays revealed that αvβ3 integrin plays a key role in intercellular communication and formation of TH2 cell clusters, with the cell surface αvβ3 integrin molecules acting like ‘Velcro’, clustering TH2 cells together. Inside these clusters, αvβ3 integrin sustains intracellular mTOR signalling to support cell growth and differentiation, forming cytokine-producing cell factories. Analogous studies using human T cells were conducted with Martin Knolle, a respiratory consultant at Cambridge University Hospitals, to reveal that shared pathways are used across mouse and human cells.
This study is the first to directly demonstrate a role for αvβ3 integrin in IL-13 production. These results indicate that inhibition of this integrin pathway may represent a novel therapeutic target for the treatment of severe asthma and allergic diseases. The work paves the way for future studies involving the role of αvβ3 integrin in human allergic diseases.
The work was funded by UKRI MRC, the Wellcome Trust, the Croucher Foundation and an MRC CARP award.
Further references
An avβ3 integrin checkpoint is critical for efficient TH2 cytokine polarisation and potentiating antigen-specific immunity. Szeto, ACH., Ferreira, ACF., Mannion, J., Clark, PA., Sivasubramaniam, M., Heycock, MWD., Crisp, A., Jolin, HE., Kozik, P., Knolle, MD., McKenzie, ANJ. Nature Immunology
Andrew McKenzie’s group page
Patrycja Kozik’s group page
Previous Insight on Research
Blocking action of intestinal immune cell enhances the immune response to colorectal cancer
How the fate of immune cell precursors is decided