Ubiquitin ligase HERC2 and adapter protein ZNRD2 are essential for efficient degradation of unassembled subunits of the cytosolic chaperonin
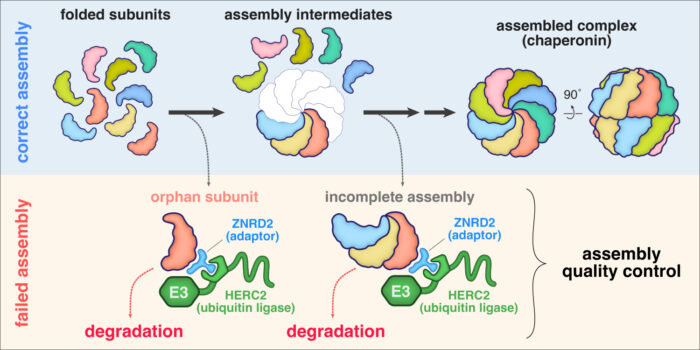
Biological processes essential for life are carried out by molecular machines often built from many protein subunits. Cells must produce these multi-subunit complexes by first synthesising each subunit, then assembling them together into the final structure. Any subunits produced in excess are “orphaned” from the final complex, whereas a subunit deficiency can lead to incomplete assemblies. It has long been known that cells eliminate orphan subunits and incomplete assemblies, but how quality control is enforced during assembly of the cell’s major molecular machines is not well understood. Manu Hegde’s group in the Cell Biology Division have now discovered how cells find and selectively tag orphan subunits of chaperonin, an abundant molecular machine responsible for protein folding.
Cytosolic chaperonin, known as CCT or TRiC, is built from two copies each of eight related subunits. Due to the imprecision of gene expression, cells rarely make all eight subunits in exactly equal amounts. To determine how orphan CCT subunits might be eliminated, Yuichi Yagita, a postdoc in Manu’s group, searched for interacting factors. With help from Sew-Yeu Peak-Chew from the mass spectrometry facility, he discovered that multiple orphan CCT subunits associate with HERC2 and ZNRD2, two little-studied proteins of unknown function. He found that ZNRD2 recognises CCT orphans and recruits HERC2, which attaches a degradation tag called ubiquitin to the orphan. Eszter Zavodszky, a senior scientist in Manu’s group, reconstituted this tagging reaction with purified ZNRD2 and HERC2, rigorously establishing their respective roles as adaptor and ubiquitin ligase. In cells, both ZNRD2 and HERC2 were essential for efficient degradation of most CCT orphans.
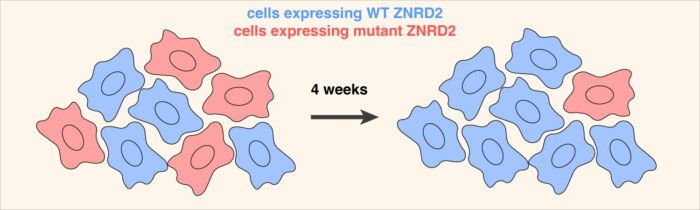
Yuichi then used AlphaFold2 to predict the structure of ZNRD2 and HERC2 in complex with an individual CCT subunit. This proved to be an accurate model as precise mutations at key interaction interfaces not only impaired the elimination of orphaned CCT subunits but also reduced the fitness of cultured cells. Additional experiments by Yuichi and Eszter found that cells are constantly producing a substantial amount of orphan CCT subunits, but these are not seen normally because ZNRD2 and HERC2 eliminate them before they accumulate.
This general process, termed assembly quality control, is of importance to human health for two reasons. First, insufficient protein quality control can lead to neurodegeneration. Indeed, loss of even one copy of HERC2 in mice causes numerous problems including neuronal death, and a HERC2 mutant impaired in its interaction with ZNRD2 has been found in a patient with neurodevelopmental problems. Assembly quality control is also especially important for the survival of cancer cells. This is because aberrant gene expression, including large imbalances in the synthesis of protein subunits, is a near-universal feature of cancer. Assembly quality control pathways that offset subunit imbalances by eliminating orphans might represent key vulnerabilities of various cancers. The pathway for eliminating orphan CCT subunits, together with an earlier assembly quality control pathway found by Manu’s group that eliminates other types of orphans, are potential therapeutic targets that warrant future investigation.
This work was funded by UKRI MRC, the Naito Foundation and the Osamu Hayaishi Memorial Scholarship for Study Abroad.
Further references
Mechanism of orphan subunit recognition during assembly quality control . Yagita, Y., Zavodszky, E., Peak-Chew, S-Y., Hegde, RS. Cell
Manu’s group page
Previous Insight on Research articles
Pathway behind disposal of ‘orphan’ proteins identified
Mechanism for cleaning up leftover proteins