Membrane curvature and presence of unsaturated lipids affect the activity of protein complexes involved in autophagy and endosomal trafficking
Lipid membranes surround our cells and form the boundaries around compartments within them. The existence of over 1000 major lipid types creates enormous chemical diversity, which can have a great impact on how proteins bind to membranes in different parts of the cell. Roger Williams’ group, in the LMB’s PNAC Division, has now shown how membrane characteristics can tune the activities of protein complexes that have important roles in autophagy, cell division, and endosomal membrane trafficking.
Membranes around different subcellular compartments display distinct properties due in part to differential enrichment of different types of lipids. This allows some membranes to be more flexible or more permeable than others. One determinant of these membrane characteristics is how tightly the lipids are able to pack together. Saturated lipids have no double bonds and their acyl chains have uniform geometries allowing many such lipids to pack together tightly, restricting flexibility. In contrast, the double bonds in the acyl chains of unsaturated lipids create kinks, so that packing is much looser.
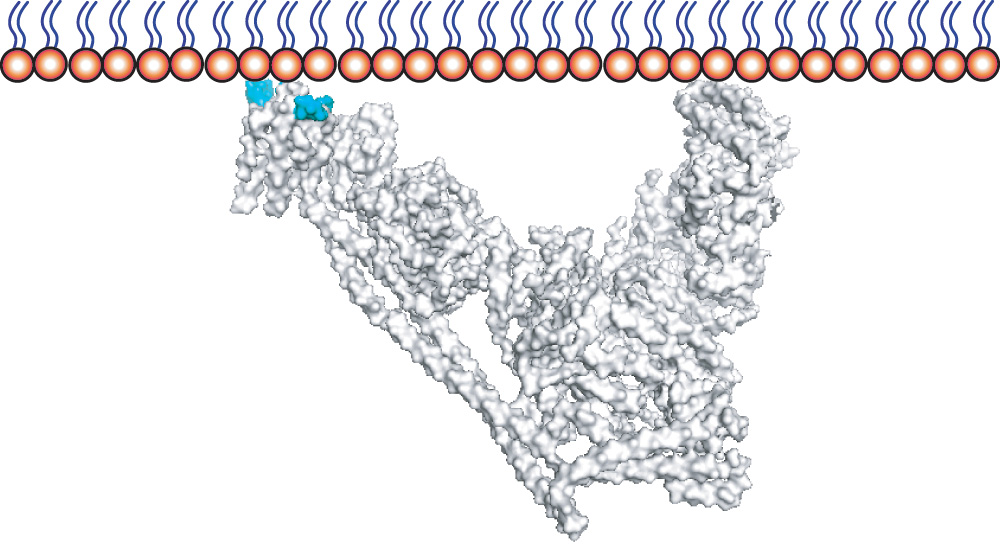
The lipid kinase VPS34 is an enzyme that, while a component of a number of distinct complexes, works at membranes to modify the headgroup of certain types of lipids to produce an important phospholipid known as PI3P (phosphatidylinositol 3-phosphate). By recruiting many different proteins, PI3P plays a critical role in a number of membrane-dependent processes, including endosomal membrane trafficking and autophagy. Roger’s group has now demonstrated the impact that membrane properties have on VPS34 activity.
To investigate how VPS34 activity is affected by different membrane compositions, Yohei Ohashi and Shirley Tremel, two members of Roger’s group, assembled the purified protein components of two different VPS34-containing complexes and created a lipid sphere that could be attached to a surface to allow easy monitoring. With support from the LMB’s Light Microscopy and Biophysics facilities and collaborators at the Babraham Institute, Yohei and Shirley were able to observe greater VPS34 activity at highly curved membranes where curvature causes packing defects that allow better protein attachment. They also found that VPS34 is activated by unsaturated lipids, whether they are the target lipid that will be modified or surrounding lipids; due, again, to packing defects that allow the protein to more easily attach itself to the membrane.
This system, and the association of VPS34 with distinct partner proteins in different locations, allows the cell to tune the activity of VPS34 at different subcellular compartments. By developing a better understanding of this control, it might be possible to design better VPS34 inhibitors and activators that could, for example, be beneficial for treatment of cancer or activation of autophagy as a therapeutic against neurodegenerative diseases.
The work was funded by UKRI MRC, Cancer Research UK, the AstraZeneca/LMB Blue Sky Initiative, the Cambridge Philosophical Society, and St Catharine’s College, Cambridge.
Further references
Membrane characteristics tune activities of endosomal and autophagic human VPS34 complexes. Ohashi, Y., Tremel, S., Masson, GR., McGinney, L., Boulanger, J., Rostislavleva, K., Johnson, CM., Niewczas, I., Clark, J., Williams, RL. eLife 9:e58281
Roger’s group page
Biological Chemistry Facility at the Babraham Institute
Previous Insight on Research
Structure of the endosomal Vps34 complex II reveals insights into its function