Inside our cells there are many distinct membrane compartments – organelles – which carry out the different tasks that allow the cell to function. Each organelle is like a factory that requires a constant supply of raw materials to stay active. Small transport vesicles deliver this cargo of particular proteins and lipids to each organelle. Like delivery trucks that must make timely shipments to a particular factory, the targeting of vesicles to exactly the right organelle is of vital importance to organelle function. To do this, specific vesicles target and bind to specific tethers on their destination organelle, before fusing with and delivering their cargo to that organelle. An organelle known as the Golgi apparatus receives vesicles from a variety of locations in the cell. The golgins, a large family of tentacle-like long coiled-coil proteins found on the Golgi apparatus, were previously shown by Sean Munro’s group in the LMB’s Cell Biology Division to function as tethers that target specific vesicles to the Golgi. New work by Sean’s group has now revealed the missing link that connects endosome-to-Golgi transport vesicles to the golgin tethers.
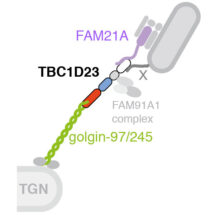
John Shin and Alison Gillingham from Sean’s group used protein engineering to relocate golgins -97 and -245 to the mitochondria, causing them to capture and permanently bind their incoming transport vesicles. The scientists then performed a proteomic technique to label all proteins in close proximity to the golgins, allowing them to detect likely proteins to which the golgins bind. This identified TBC1D23, a protein of otherwise unknown function. Further experiments revealed that the N-terminus of TBC1D23 binds to the tip of golgin-97 and golgin-245 at the trans-Golgi, while its C-terminus binds to the WASH complex on endosome-derived vesicles. In addition, they found that the FAM91A1 complex, whose function is also unknown, binds to TBC1D23 at a site adjacent to its vesicle binding region, suggesting FAM91A1 may regulate vesicle binding.
This is the first paper to identify a protein, TBC1D23, that connects a specific pool of transport vesicles to specific golgins. It provides a roadmap for future identification of other vesicle cues that recognise specific tethers. In addition, this study is the first to show that a TBC protein can function as an adaptor in vesicle tethering. There are many other TBC proteins and it is possible that these too play similar roles as tethering adapters. Importantly, TBC1D23 has been implicated in the regulation of the innate immune system in mice and homozygous mutation of TBC1D23 is a cause of pontocerebellar hypoplasia and reduced cortical development in humans. Thus, this work provides a possible mechanistic insight into the causes of these disorders, which will be crucial to the development of future treatments.
This work was funded by the MRC and a European Molecular Biology Organisation Fellowship.