Structure of the outer kinetochore bound to microtubules reveals how phosphorylation regulates mitotic spindle chromosome attachment errors to ensure DNA is equally segregated into two daughter cells
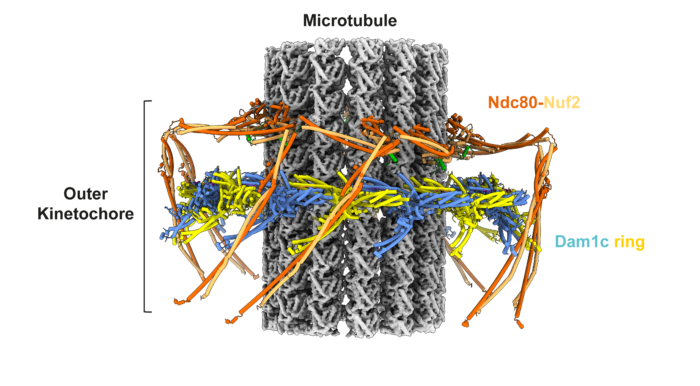
Kinetochores are large molecular machines that attach chromosomes to the mitotic spindle. A kinetochore-mediated error correction mechanism ensures chromosomes are correctly bi-oriented so that DNA is equally segregated into two daughter cells, which is essential for genome integrity. However, the molecular mechanics of how this process works and is regulated has remained an unresolved question. Now, David Barford’s group, in the LMB Structural Studies Division, describes the electron cryo-microscopy (cryo-EM) structure of the budding yeast outer kinetochore bound to microtubules. The structure reveals how phosphorylation regulates kinetochore-microtubule attachments during mitotic error correction.
The kinetochore consists of two multi-protein complexes: the inner kinetochore binds to centromeres via a mechanism described previously by the Barford group, whilst the outer kinetochore attaches to mitotic spindle microtubules which pull the sister chromosomes apart into two daughter cells during mitosis. To ensure sister chromosomes are correctly attached to mitotic spindles an error correction pathway drives the turnover of outer kinetochore-microtubule attachments until biorientation is achieved. This process is regulated by phosphorylation of outer kinetochore subunits in response to low tension at the kinetochore-microtubule interface. Low tension signals lack of bi-polar attachment of the sister kinetochore to the mitotic spindle.
To gain deeper mechanistic understanding of the mitotic error correction pathway, the team, led by Kyle Muir, a postdoc in the Barford group, reconstituted and determined the cryo-EM structure of the budding yeast outer kinetochore-microtubule complex. The structure reveals multiple interfaces between components of the complex. The presence of error-correction phosphorylation sites within these key protein interfaces indicates why error-correction would drive both outer kinetochore disassembly and destabilise attachment to microtubules.
To further investigate this, the team used optical tweezers to measure the force required to break apart kinetochore-microtubule attachments and found that mutating protein interfaces between the outer kinetochore components reduced attachment strength. Mutations that showed substantial reduction in attachment strength also led to cell death, showing that outer kinetochore assembly interfaces observed in the structure are also important for cell viability.
The team propose a model in which phosphorylated and disassembled outer kinetochore components diffuse away from the microtubule and are replaced by unphosphorylated components. This allows new attachments to form even under initially low-tension conditions, thus resolving the paradox of how kinetochore-microtubule connections can reset under low-tension conditions.
Proteins involved in regulating mitotic error correction checkpoints are targets of anti-cancer drugs. Understanding this process at a molecular level should provide further insights into the molecular basis of the targets of these anti-cancer drugs.
This work was funded by UKRI MRC and Cancer Research UK.
Further references
Structural mechanism of outer kinetochore Dam1:Ndc80 complex assembly on microtubules. Muir, K.W., Batters, C., Dendooven, T., Yang, J., Zhang, Z., Burt, A., Barford, D. Science
David’s group page
Previous Insight on Research articles
Human inner kinetochore structure reveals mechanism for binding DNA during mitosis
How chromosomes are bound to be separated in cell division